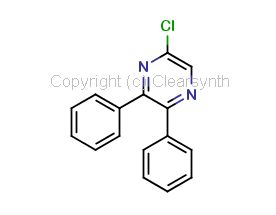
Selexipag Impurity C
| Product Name | Selexipag Impurity C |
|---|---|
| Alternate Names | Selexipag Impurities, Impurities of Selexipag |
| CAT No. | CS-AQ-00230 |
| CAS No. | 41270-66-0 |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | 266.72 g/mol |
| Mol. For. | C₁₆H₁₁ClN₂ |
| Hazardous | This is not a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Selexipag |
| Purity | 95% |
| Smileys | ClC1=CN=C(C2=CC=CC=C2)C(C3=CC=CC=C3)=N1 |
| Canonical Smiles | C1=CC=C(C=C1)C2=NC=C(N=C2C3=CC=CC=C3)Cl |
| InchIKey | VUGNCPVAXWZTOL-UHFFFAOYSA-N |
| Inchl | InChI=1S/C16H11ClN2/c17-14-11-18-15(12-7-3-1-4-8-12)16(19-14)13-9-5-2-6-10-13/h1-11H |
| IUPAC | 5-chloro-2,3-diphenylpyrazine |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Selexipag Impurity C is a byproduct formed during the synthesis of the drug Selexipag. Selexipag is a medication used for the treatment of pulmonary arterial hypertension. It works by activating the prostacyclin receptor, which leads to the dilation of blood vessels and a reduction in blood pressure. Selexipag Impurity C, on the other hand, is an impurity that is produced during the manufacturing process of Selexipag.
The chemical structure of Selexipag Impurity C is closely related to that of Selexipag. It is a derivative of the same chemical compound and contains the same functional groups. However, it is not intended for use in the treatment of any medical condition. Instead, it is used as a reference standard during the quality control process of Selexipag.
Selexipag Impurity C is used in the pharmaceutical industry to monitor the purity of Selexipag. It is necessary to ensure that Selexipag is free from any impurities that may be harmful to patients. The presence of Selexipag Impurity C is detected and quantified using analytical techniques such as high-performance liquid chromatography (HPLC).
Overall, Selexipag Impurity C is an important reference standard used in the quality control process of Selexipag. Its chemical structure closely resembles that of Selexipag and it is used to ensure that Selexipag is of high purity and safe for use in patients.
Get an Instant Quote
Related Compounds
Sexipag Glycine Adduct | Selexipag N-Oxide | Selexipag N1-Oxide | Selexipag Impurity 28 | Selexipag O - Impurity | Selexipag Impurity 9 | Selexipag Impurity A | Selexipag DPB Desisopropyl Impurity | Selexipag Impurity B | Selexipag Mannitol Ester | Selexipag formyl impurity | Selexipag Impurity at RT about 42.3 | Selexipag Impurity D | Selexipag Dimer Impurity | Selexipag Methyl Ester Impurity |