Atorvastatin sodium salt 13C6
| Chemical Name | Atorvastatin sodium salt 13C6 |
|---|---|
| Alternate Names | Atorvastatin Stable Isotopes, Stable Isotopes of Atorvastatin |
| CAT No. | CS-EK-02903 |
| CAS Registry# | 134523-01-6 (Unlabelled) |
| Category | Stable Isotopes |
| Stock | Enquire |
| Mol. Wt. | Not Available |
| Mol. For. | Not Available |
| Hazardous | This is not a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Atorvastatin |
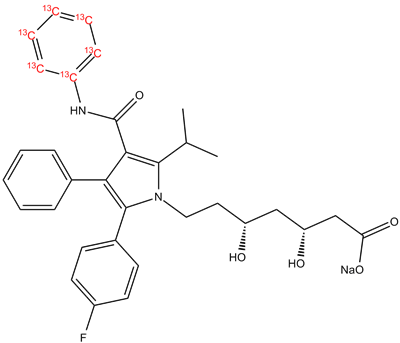
| Canonical Smiles | CC(C)C1=C(C(=C(N1CCC(CC(CC(=O)[O-])O)O)C2=CC=C(C=C2)F)C3=CC=CC=C3)C(=O)NC4=CC=CC=C4.[Na+] |
| InchIKey | VVRPOCPLIUDBSA-CNZCJKERSA-M |
| Inchl | InChI=1S/C33H35FN2O5.Na/c1-21(2)31-30(33(41)35-25-11-7-4-8-12-25)29(22-9-5-3-6-10-22)32(23-13-15-24(34)16-14-23)36(31)18-17-26(37)19-27(38)20-28(39)40;/h3-16,21,26-27,37-38H,17-20H2,1-2H3,(H,35,41)(H,39,40);/q;+1/p-1/t26-,27-;/m1./s1 |
| IUPAC | sodium;(3R,5R)-7-[2-(4-fluorophenyl)-3-phenyl-4-(phenylcarbamoyl)-5-propan-2-ylpyrrol-1-yl]-3,5-dihydroxyheptanoate |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
[13C6]-Atorvastatin sodium salt is a chemically modified form of Atorvastatin, a medication used to lower cholesterol levels in the body. Atorvastatin is a type of statin, which works by inhibiting the production of cholesterol in the liver. It is commonly prescribed to patients with high levels of low-density lipoprotein (LDL) cholesterol, also known as "bad" cholesterol.
[13C6]-Atorvastatin sodium salt is labeled with six carbon-13 atoms, which can be used in research studies to track the metabolism and pharmacokinetics of Atorvastatin. This label makes it possible to distinguish between Atorvastatin and other compounds in biological samples using mass spectrometry techniques.
The chemical formula of [13C6]-Atorvastatin sodium salt is C33H34FN2NaO5[13CH3]6, with a molecular weight of 798.9 g/mol. It is a white to off-white powder that is soluble in water and ethanol.
The recommended dose of Atorvastatin varies depending on the patient's age, gender, and medical history. It is typically taken once daily, either with or without food. Common side effects of Atorvastatin include muscle pain, nausea, and diarrhea. In rare cases, it can cause liver damage or rhabdomyolysis, a severe muscle injury.
Overall, [13C6]-Atorvastatin sodium salt is a valuable tool for researchers investigating the pharmacokinetics and metabolism of Atorvastatin. Its unique labeling allows for precise detection and quantification of the compound in biological samples, leading to a better understanding of its effects on the body.
Get an Instant Quote
Related Compounds
Atorvastatin D5 calcium | Atorvastatin D5 sodium | p-Hydroxyatorvastatin 13C6 | Atorvastatin D5 | Atorvastatin-d5 (N-(phenyl-d5) Lactone | Atorvastatin D5 4-(phenyl-d5) Lactone |