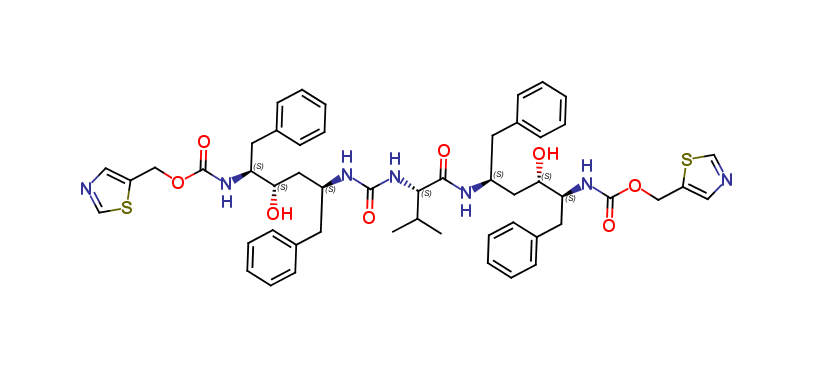
Ritonavir EP Impurity S
| Product Name | Ritonavir EP Impurity S |
|---|---|
| Alternate Names | Ritonavir Impurities, Impurities of Ritonavir |
| CAT No. | CS-EO-00169 |
| CAS No. | 2084828-53-3 |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | 976.21 g/mol |
| Mol. For. | C₅₂H₆₁N₇O₈S₂ |
| Hazardous | This is not a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Ritonavir |
| Smileys | O[C@H]([C@@H](NC(OCC1=CN=CS1)=O)CC2=CC=CC=C2)C[C@@H](NC([C@H](C(C)C)NC(N[C@H](C[C@H](O)[C@@H](NC(OCC3=CN=CS3)=O)CC4=CC=CC=C4)CC5=CC=CC=C5)=O)=O)CC6=CC=CC=C6 |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Ritonavir is an antiretroviral drug used in the treatment of HIV/AIDS. Ritonavir EP Impurity S is a chemical compound that is used as a reference standard in the pharmaceutical industry to determine the purity and quality of Ritonavir. It is a synthetic compound and its chemical name is (3R,4R,6S)-4-hydroxy-6-[(1R)-1-hydroxyethyl]-3-methyl-2-oxoheptanenitrile.
The usage of Ritonavir EP Impurity S is primarily in the pharmaceutical industry as a quality control measure. It is used in the development and manufacture of Ritonavir-based drugs to ensure that the final product is of high quality and free from impurities. The impurity level of drugs is strictly regulated, and the use of Ritonavir EP Impurity S helps to ensure that the impurity levels are within acceptable limits.
Chemically, Ritonavir EP Impurity S is a nitrile compound with a molecular weight of 231.3 g/mol. It is a white to off-white crystalline powder that is soluble in water, ethanol, and methanol. It is stable under normal conditions of storage and handling.
In conclusion, Ritonavir EP Impurity S is an important reference standard in the pharmaceutical industry that is used to ensure the quality and purity of Ritonavir-based drugs. It is a synthetic nitrile compound with a molecular weight of 231.3 g/mol and is soluble in water, ethanol, and methanol. It plays a crucial role in the development and manufacture of Ritonavir-based drugs, ensuring that they are safe and effective for use in the treatment of HIV/AIDS.
Get an Instant Quote
Related Compounds
Ritonavir Hydroxypropyl carbamate | Ritonavir 2,5-Thiazoylmethyl-Dicarbamate | Ritonavir Geo-isomer Hcl | S-(1-methyl-1H-imidazol-4-yl) 2-methylpropanethioate | thiazol-5-ylmethyl ((2S,3S,5S)-5-((S)-2-(3-((2-(2-hydroperoxypropan-2-yl)thiazol-4-yl)methyl)-3-methylureido)-3-methylbutanamido)-3-hydroxy-1,6-diphenylhexan-2-yl)carbamate | Ritonavir EP Impurity I | Ritonavir Enantiomer | Ritonavir Impurity U | O-Acetyl Ritonavir | Ritonavir Impurity 30 | Ritonavir Hydrogen Peroxide Impurity | thiazol-5-ylmethyl ((2S,3S,5S)-3-hydroxy-5-((R)-2-(3-((5-(2-hydroxypropan-2-yl)thiazol-2-yl)methyl)- | Ritonavir Impurity 27 | Ritonavir Impurity S | Ritonavir EP Impurity O | Ritonavir EP Impurity D | Ritonavir EP Impurity Q | Ritonavir EP Impurity I | Thiazol-5-ylmethyl ((2S, 3S, 5S)-3-hydroxy-5-(2-(3-((5-isopropylthiazol-2-yl) methyl)-3-methylureido | Ritonavir EP Impurity M | Ritonavir Hydroxy impurity | Des(isopropylthiazolyl)-N-methyl Ritonavir | Ritonavir EP Impurity F | Ritonavir EP Impurity E | Ritonavir EP Impurity N | Ritonavir Impurity T | Ritonavir EP Impurity K | Ritonavir Impurity 22 | Ritonavir Acid Impurity | Ritonavir Impurity G | Ritonavir-Glycerol carbamate Analog | Ritonavir EP Impurity U | Ritonavir EP Impurity R | Ritonavir Impurity 23 | Ritonavir Geo-isomer | Ritonavir EP Impurity P |