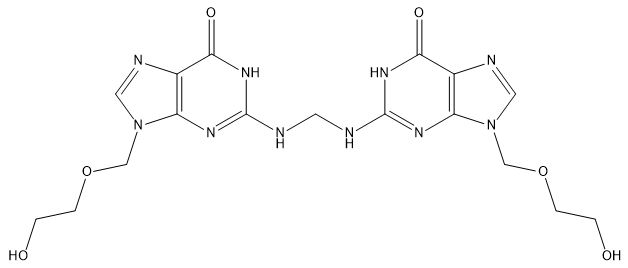
Aciclovir Impurity K
| Product Name | Aciclovir Impurity K |
|---|---|
| CAT No. | CS-J-00012 |
| CAS No. | 1797131-64-6 |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | 462.42 g/mol |
| Mol. For. | C₁₇H₂₂N₁₀O₆ |
| Hazardous | This is not a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Canonical Smiles | C1=NC2=C(N1COCCO)N=C(NC2=O)NCNC3=NC4=C(C(=O)N3)N=CN4COCCO |
| InchIKey | DHOYYKLYLLHMRJ-UHFFFAOYSA-N |
| Inchl | InChI=1S/C17H22N10O6/c28-1-3-32-8-26-6-20-10-12(26)22-16(24-14(10)30)18-5-19-17-23-13-11(15(31)25-17)21-7-27(13)9-33-4-2-29/h6-7,28-29H,1-5,8-9H2,(H2,18,22,24,30)(H2,19,23,25,31) |
| IUPAC | 9-(2-hydroxyethoxymethyl)-2-[[[9-(2-hydroxyethoxymethyl)-6-oxo-1H-purin-2-yl]amino]methylamino]-1H-purin-6-one |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Aciclovir Impurity K is a chemical impurity that is commonly found in aciclovir, a well-known antiviral medication used to treat herpes simplex virus infections. Aciclovir Impurity K is a byproduct of the manufacturing process of aciclovir and is typically present in small amounts in aciclovir formulations.
The usage of Aciclovir Impurity K is primarily as a reference standard for the identification and quantification of impurities in aciclovir formulations. It is also used in the development of analytical methods to detect and measure impurities in aciclovir.
From a chemical standpoint, Aciclovir Impurity K is a complex organic compound with a molecular weight of 261.32 g/mol. Its chemical formula is C14H19N3O3, and it is classified as a pyrimidine nucleoside analogue.
The presence of Aciclovir Impurity K in aciclovir formulations is closely monitored by regulatory authorities to ensure that the levels are within acceptable limits. This is because the presence of impurities in pharmaceutical products can potentially affect their safety, efficacy, and quality.
In conclusion, Aciclovir Impurity K is a chemical impurity that is important in the analysis and quality control of aciclovir formulations. Its usage as a reference standard ensures that aciclovir medications are safe and effective for patients.
Get an Instant Quote
This page contains information about Aciclovir Impurity K. You can buy Aciclovir Impurity K from Clearsynth at best competitive price with assured price guarantee. Clearsynth offers best quality Aciclovir Impurity K