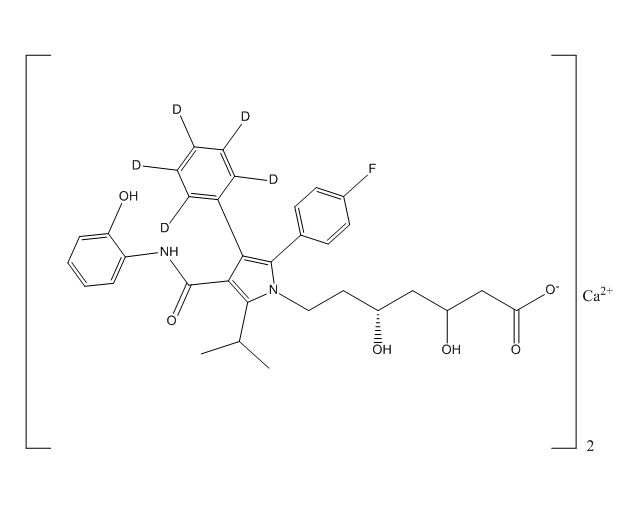
2-Hydroxy Atorvastatin D5 Calcium Salt
| Chemical Name | 2-Hydroxy Atorvastatin D5 Calcium Salt |
|---|---|
| Alternate Names | Atorvastatin Stable Isotopes, Stable Isotopes of Atorvastatin |
| CAT No. | CS-O-00659 |
| CAS Registry# | 265989-46-6(Unlabeled) |
| Category | Stable Isotopes |
| Stock | IN-Stock |
| Mol. Wt. | 1197.40 g/mol |
| Mol. For. | C₆₆H₅₈D₁₀CaF₂N₄O₁₂ |
| Hazardous | This is a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Atorvastatin |
| Purity | 95% |
| Therapeutic | Anti-Diabetic |
| Canonical Smiles | CC(C)C1=C(C(=C(N1CCC(CC(CC(=O)[O-])O)O)C2=CC=C(C=C2)F)C3=CC=CC=C3)C(=O)NC4=CC=CC=C4O.CC(C)C1=C(C(=C(N1CCC(CC(CC(=O)[O-])O)O)C2=CC=C(C=C2)F)C3=CC=CC=C3)C(=O)NC4=CC=CC=C4O.[Ca+2] |
| InchIKey | NOCWNJZXNVSDOU-UHFFFAOYSA-L |
| Inchl | InChI=1S/2C33H35FN2O6.Ca/c2*1-20(2)31-30(33(42)35-26-10-6-7-11-27(26)39)29(21-8-4-3-5-9-21)32(22-12-14-23(34)15-13-22)36(31)17-16-24(37)18-25(38)19-28(40)41;/h2*3-15,20,24-25,37-39H,16-19H2,1-2H3,(H,35,42)(H,40,41);/q;;+2/p-2 |
| IUPAC | calcium;7-[2-(4-fluorophenyl)-4-[(2-hydroxyphenyl)carbamoyl]-3-phenyl-5-propan-2-ylpyrrol-1-yl]-3,5-dihydroxyheptanoate |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
2-Hydroxy Atorvastatin D5 Calcium Salt is a chemical compound that belongs to the class of statins, which are drugs used to lower cholesterol levels in the body. This compound is a deuterated derivative of atorvastatin calcium and contains five deuterium atoms. It is also known as (3R,5R)-7-[2-(4-fluorophenyl)-3-phenyl-4-(phenylcarbamoyl)-5-propan-2-ylpyrrol-1-yl]-3,5-dihydroxyheptanoic acid calcium salt.
The usage of 2-Hydroxy Atorvastatin D5 Calcium Salt is primarily for research and analytical purposes. It is used as a reference standard in the development and validation of analytical methods for the quantification of atorvastatin in biological samples. It is also used in the study of the pharmacokinetics and metabolism of atorvastatin in the body.
The chemical information of this compound includes its molecular weight, which is 1207.22 g/mol, and its molecular formula, which is C66H73D5CaFNO13. It is a white to off-white powder that is soluble in water and ethanol. The compound is stable under normal storage conditions and can be stored at room temperature. However, it should be protected from light and moisture to prevent degradation.
In conclusion, 2-Hydroxy Atorvastatin D5 Calcium Salt is a useful tool for researchers studying atorvastatin metabolism and pharmacokinetics. Its high purity and stability make it an ideal reference standard for the development of analytical methods for atorvastatin quantification.
Get an Instant Quote
Related Compounds
p-Hydroxyatorvastatin 13C6 | Atorvastatin D5 4-(phenyl-d5) Lactone | Atorvastatin D5 sodium | Atorvastatin sodium salt 13C6 | Atorvastatin-d5 (N-(phenyl-d5) Lactone | Atorvastatin D5 | Atorvastatin D5 calcium |