Atorvastatin D5 calcium
| Product Name | Atorvastatin D5 calcium |
|---|---|
| Alternate Names | Atorvastatin Stable Isotopes, Stable Isotopes of Atorvastatin |
| CAT No. | CS-O-01265 |
| CAS No. | 222412-82-0 |
| Category | Stable Isotopes |
| Stock | IN-Stock |
| Mol. Wt. | 1165.42 g/mol |
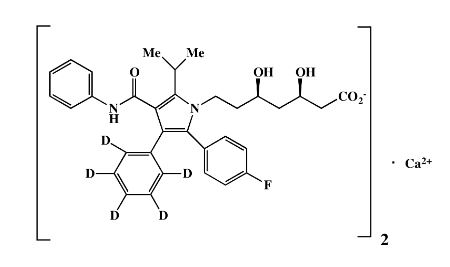
| Mol. For. | C₆₆H₅₈D₁₀CaF₂N₄O₁₀ |
| Hazardous | This is a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Atorvastatin |
| Purity | 95% |
| Therapeutic | Anti-Diabetic |
| Smileys | CC(C)C1=C(C(=C(N1CCC(CC(CC(=O)[O-])O)O)C2=CC=C(C=C2)F)C3=CC=CC=C3)C(=O)NC4=CC=CC=C4.[Ca+2] |
| Canonical Smiles | CC(C)C1=C(C(=C(N1CCC(CC(CC(=O)[O-])O)O)C2=CC=C(C=C2)F)C3=CC=CC=C3)C(=O)NC4=CC=CC=C4.[Ca+2] |
| InchIKey | DYJKAHNDTTZSNX-ADFDHUHVSA-M |
| Inchl | InChI=1S/C33H35FN2O5.Ca/c1-21(2)31-30(33(41)35-25-11-7-4-8-12-25)29(22-9-5-3-6-10-22)32(23-13-15-24(34)16-14-23)36(31)18-17-26(37)19-27(38)20-28(39)40;/h3-16,21,26-27,37-38H,17-20H2,1-2H3,(H,35,41)(H,39,40);/q;+2/p-1/t26-,27-;/m1./s1/i3D,5D,6D,9D,10D; |
| IUPAC | calcium;(3R,5R)-7-[2-(4-fluorophenyl)-3-(2,3,4,5,6-pentadeuteriophenyl)-4-(phenylcarbamoyl)-5-propan-2-ylpyrrol-1-yl]-3,5-dihydroxyheptanoate |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Atorvastatin D5 calcium is a pharmaceutical drug that is used to lower cholesterol levels in the blood. It belongs to a class of drugs known as statins and is prescribed to individuals who have high levels of low-density lipoprotein (LDL) cholesterol or are at risk of developing cardiovascular diseases such as heart attacks and strokes. Atorvastatin D5 calcium works by inhibiting the activity of an enzyme known as HMG-CoA reductase, which is responsible for the production of cholesterol in the liver.
The chemical name of Atorvastatin D5 calcium is (3R,5R)-7-[2-(4-fluorophenyl)-3-phenyl-4-(phenylcarbamoyl)-5-propan-2-ylpyrrol-1-yl]-3,5-dihydroxyheptanoic acid calcium salt pentahydrate. Its molecular formula is C33H34FNO5Ca•5H2O, and its molecular weight is 729.1 g/mol. The drug is available in tablet form, with dosages ranging from 10mg to 80mg.
Atorvastatin D5 calcium should be taken as directed by a healthcare provider and is usually taken once daily, with or without food. The drug should not be taken by individuals who are allergic to any of its ingredients or have liver problems. Side effects of Atorvastatin D5 calcium may include muscle pain, weakness, and liver problems. It is important to notify a healthcare provider if any of these side effects occur.
Get an Instant Quote
Related Compounds
Atorvastatin D5 sodium | Atorvastatin sodium salt 13C6 | Atorvastatin D5 | Atorvastatin-d5 (N-(phenyl-d5) Lactone | Atorvastatin D5 4-(phenyl-d5) Lactone | p-Hydroxyatorvastatin 13C6 |