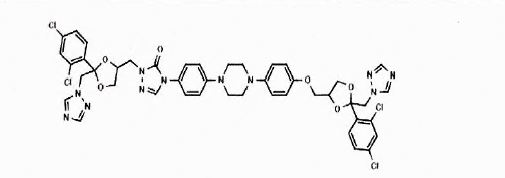
Itraconazole EP Impurity G
| Product Name | Itraconazole EP Impurity G |
|---|---|
| Alternate Names | Itraconazole Impurities, Impurities of Itraconazole |
| CAT No. | CS-O-03574 |
| CAS No. | Not Available |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | 961.68 g/mol |
| Mol. For. | C₄₄H₄₁Cl₄N₁₁O₆ |
| Hazardous | This is a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Itraconazole |
| Purity | 95% |
| Therapeutic | Anti-Fungals |
| Smileys | ClC1=C([C@]2(CN3C=NC=N3)O[C@H](CO2)COC4=CC=C(C=C4)N5CCN(CC5)C6=CC=C(C=C6)N(C7=O)C=NN7CC8OC(C9=C(Cl)C=C(Cl)C=C9)(CN%10C=NC=N%10)OC8)C=CC(Cl)=C1 |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Itraconazole EP Impurity G is a chemical compound that is commonly used in pharmaceutical research and development. It is a potential impurity of Itraconazole, which is an antifungal medication used to treat various fungal infections. Itraconazole EP Impurity G is a by-product that may be formed during the manufacturing process of Itraconazole, and it has been identified as an impurity that may be present in Itraconazole drug products.
Chemically, Itraconazole EP Impurity G is an organic compound with a molecular formula of C35H45NO4. It has a molecular weight of 557.74 g/mol and a melting point range of 202-204°C. The chemical structure of Itraconazole EP Impurity G includes a quinoline ring system, which is a common structural feature of many antifungal agents.
The usage of Itraconazole EP Impurity G is mainly for analytical purposes. It is used as a reference standard in the development and validation of analytical methods for the determination of impurities in Itraconazole drug products. It is also used as a reference material in the characterization and identification of Itraconazole impurities.
In summary, Itraconazole EP Impurity G is an important reference standard in pharmaceutical research and development. Its chemical information and usage are crucial for the development and validation of analytical methods for the determination of impurities in Itraconazole drug products.
Get an Instant Quote
Related Compounds
ITRACONAZOLE IMPURITY 18 | ITRACONAZOLE IMPURITY 19 | itraconazole 4-Triazolyl isomer | Itraconazole EP Impurity C | ITRACONAZOLE RELATED IMPURITY 3 | Itraconazole Desethylene-seco-piperazine Di-N-formyl Impurity | itraconazole n-Butyl isomer | Itraconazole Hydroxy Butyltriazolone Impurity | Itraconazole Methoxy Isopropyltriazolone Impurity | Itraconazole Methoxy Propyltriazolone Impurity | Itraconazole EP Impurity E | ITRACONAZOLE n-propyl impurity | Itraconazole EP Impurity B | Itraconazole Hydroxy Propyltriazolone Impurity | Itraconazole S,R IMPURITY | Itraconazole Impurity 7 | ITRACONAZOLE IMPURITY 11 | ITRACONAZOLE IMPURITY 13 | Itraconazole EP Impurity D | Itraconazole Impurity 2 | Itraconazole Methoxy Hydrazinyl Impurity | Itraconazole EP Impurity F | Itraconazole Didioxolanyl analog | N-Nitroso Itraconazole Impurity 1 | Itraconazole EP Impurity A | ITRACONAZOLE RELATED IMPURITY 1 | Itraconazole Hydroxy Isopropyltriazolone Impurity | Itraconazole Epimer |