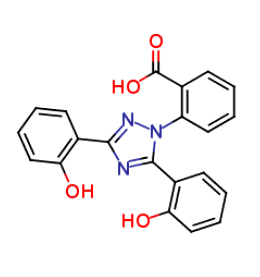
Deferasirox Phase 1 impurity
| Product Name | Deferasirox Phase 1 impurity |
|---|---|
| Alternate Names | Deferasirox Impurities, Impurities of Deferasirox |
| CAT No. | CS-O-05579 |
| CAS No. | 201530-78-1 |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | 373.36 g/mol |
| Mol. For. | C₂₁H₁₅N₃O₄ |
| Hazardous | This is not a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Deferasirox |
| Purity | >98% |
| Smileys | O=C(O)C1=CC=CC=C1N2N=C(C3=CC=CC=C3O)N=C2C4=CC=CC=C4O |
| Canonical Smiles | C1=CC=C(C(=C1)C2=NN(C(=N2)C3=CC=CC=C3O)C4=CC=CC=C4C(=O)O)O |
| InchIKey | PDBKAEPAMFPDAZ-UHFFFAOYSA-N |
| Inchl | InChI=1S/C21H15N3O4/c25-17-11-5-2-8-14(17)19-22-20(15-9-3-6-12-18(15)26)24(23-19)16-10-4-1-7-13(16)21(27)28/h1-12,25-26H,(H,27,28) |
| IUPAC | 2-[3,5-bis(2-hydroxyphenyl)-1,2,4-triazol-1-yl]benzoic acid |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Deferasirox is an iron-chelating agent used in the treatment of chronic iron overload caused by blood transfusions in patients with thalassemia and other chronic anemias. The drug works by binding to excess iron in the blood and removing it from the body through urine and feces. During the production of Deferasirox, impurities may be formed that can affect the purity and quality of the final product.
One such impurity is the Deferasirox Phase 1 impurity. This impurity is a by-product formed during the manufacturing process of Deferasirox and can be present in trace amounts in the final product. The chemical structure of this impurity is not disclosed by the manufacturer due to proprietary reasons.
The presence of Deferasirox Phase 1 impurity in the final product is monitored and controlled by regulatory agencies to ensure the safety and efficacy of the drug. The acceptable limit for this impurity is set at less than 0.1% of the total drug substance. Any amount above this limit can lead to adverse effects and may impact the therapeutic efficacy of the drug.
In conclusion, the Deferasirox Phase 1 impurity is a by-product formed during the manufacturing process of Deferasirox, which can affect the purity and quality of the final product. The presence of this impurity is monitored and controlled to ensure the safety and efficacy of the drug.
Get an Instant Quote
Related Compounds
Deferasirox Impurity 25 | Deferasirox Impurity 3 | Deferasirox bis salicylamide impurity | Deferasirox Impurity F | Deferasirox Diacyl Impurity | Deferasirox Ethyl Ester | Deferasirox Impurity D | Deferasirox Impurity 9 | Azobenzene-4,4'-dicarboxylic Acid | Deferasirox Impurity 22 | Deferasirox Impurity 3 | Deferasirox Salicyloyl Ester | Deferasirox Amide Impurity | Deferasirox Impurity 4 | Deferasirox Hydrazino Impurity | Deferasirox Isopropyl Ester |