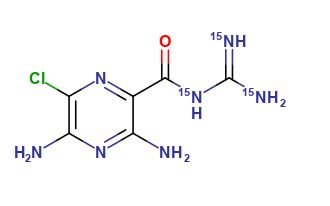
Amiloride 15N3
| Chemical Name | Amiloride 15N3 |
|---|---|
| Alternate Names | Amiloride Stable Isotopes, Stable Isotopes of Amiloride |
| CAT No. | CS-O-06400 |
| CAS Registry# | 1217169-93-1 |
| Category | Stable Isotopes |
| Stock | IN-Stock |
| Mol. Wt. | 232.607 g/mol |
| Mol. For. | C6H8ClN415N3O |
| Hazardous | This is not a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Amiloride |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Amiloride 15N3 is a medication that is primarily used to treat high blood pressure and congestive heart failure. It belongs to a class of drugs known as potassium-sparing diuretics. Amiloride 15N3 works by blocking the reabsorption of sodium ions in the kidneys, which leads to increased excretion of sodium and water from the body. This, in turn, reduces the amount of fluid in the blood vessels and helps to lower blood pressure.
The chemical formula of Amiloride 15N3 is C6H8ClN7O, and it is a nitrogen-15 labeled version of the drug amiloride. It is an odorless, white crystalline powder that is soluble in water. The molecular weight of Amiloride 15N3 is 236.68 g/mol.
Amiloride 15N3 is typically taken orally once or twice a day, with or without food. The dosage will depend on the individual's medical condition and response to treatment. Patients should not exceed the prescribed dose or take the medication for longer than recommended.
Like all medications, Amiloride 15N3 may cause side effects, such as dizziness, headache, nausea, vomiting, and diarrhea. Patients should contact their healthcare provider if they experience any severe or persistent side effects.
In conclusion, Amiloride 15N3 is a medication that is widely used for the treatment of high blood pressure and congestive heart failure. It is a potassium-sparing diuretic that works by blocking the reabsorption of sodium ions in the kidneys. While it can be an effective treatment, patients should always follow the dosage instructions provided by their healthcare provider and report any side effects they experience.
Get an Instant Quote
Related Compounds
Amiloride 15N3 Hydrochloride |