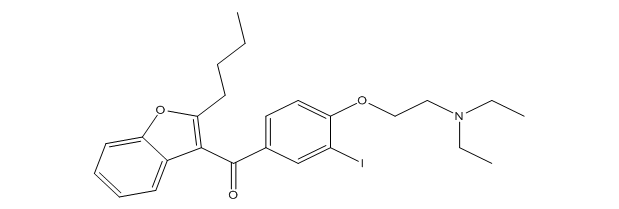
Amiodarone EP Impurity C
| Product Name | Amiodarone EP Impurity C |
|---|---|
| Alternate Names | Amiodarone Impurities, Impurities of Amiodarone |
| CAT No. | CS-O-07160 |
| CAS No. | 85642-08-6 |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | 519.42 g/mol |
| Mol. For. | C₂₅H₃₀INO₃ |
| Hazardous | This is a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Amiodarone |
| Purity | 95% |
| Canonical Smiles | CCCCC1=C(C2=CC=CC=C2O1)C(=O)C3=CC(=C(C=C3)OCCN(CC)CC)I |
| InchIKey | DKWLFFXBYKTKRM-UHFFFAOYSA-N |
| Inchl | InChI=1S/C25H30INO3/c1-4-7-11-23-24(19-10-8-9-12-21(19)30-23)25(28)18-13-14-22(20(26)17-18)29-16-15-27(5-2)6-3/h8-10,12-14,17H,4-7,11,15-16H2,1-3H3 |
| IUPAC | (2-butyl-1-benzofuran-3-yl)-[4-[2-(diethylamino)ethoxy]-3-iodophenyl]methanone |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Amiodarone EP Impurity C is an organic compound, also known as 2-Butyl-3-(3,5-diiodo-4-hydroxybenzoyl)benzofuran. It is a known impurity in the pharmaceutical drug Amiodarone, which is used to treat heart rhythm disorders.
Amiodarone EP Impurity C is formed during the synthesis of Amiodarone and may be present in small quantities in the final product. While it does not have any therapeutic value, it is important to monitor its presence as it may affect the safety and efficacy of Amiodarone.
The chemical structure of Amiodarone EP Impurity C consists of a benzofuran ring with a butyl chain and a hydroxybenzoyl group attached to it. It is a highly crystalline compound that is sparingly soluble in water and soluble in organic solvents such as methanol and acetonitrile.
It is important to note that Amiodarone EP Impurity C has not been fully characterized, and its toxicity and potential side effects are not well understood. Therefore, it is important to control its levels in Amiodarone and monitor its presence during the drug's production and storage.
In conclusion, Amiodarone EP Impurity C is an impurity that must be carefully monitored during the production of Amiodarone to ensure the safety and efficacy of the final product. Its chemical structure and properties must be understood to properly control its levels and ensure patient safety.
Get an Instant Quote
Related Compounds
Amiodarone EP Impurity A | Amiodarone EP Impurity G | Amiodarone EP Impurity D | Amidodarone HCl impurity E | Amiodarone Methoxy Impurity | N-Desethyl N-Nitroso Amiodarone | Amiodarone EP Impurity F | Des-O-[2-(diethylamino)ethyl]-1-methoxy Amiodarone | N,N-Di-desethyl Amiodarone Hydrochloride | Amiodarone EP Impurity C HCl | Amiodarone EP Impurity H | Des(diethylaminoethyl)-didesiodo-1'-methoxy Amiodarone | Amiodarone EP Impurity G(HCl salt form) |