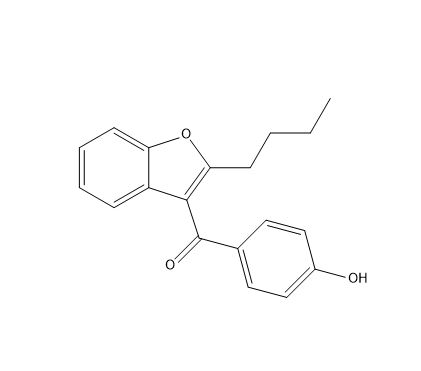
Amidodarone HCl impurity E
| Product Name | Amidodarone HCl impurity E |
|---|---|
| Alternate Names | Amiodarone Impurities, Impurities of Amiodarone |
| CAT No. | CS-O-07162 |
| CAS No. | 52490-15-0 |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | 294.34 g/mol |
| Mol. For. | C₁₉H₁₈O₃ |
| Hazardous | This is a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Amiodarone |
| Purity | >98% |
| Canonical Smiles | CCCCC1=C(C2=CC=CC=C2O1)C(=O)C3=CC=C(C=C3)O |
| InchIKey | ZHGKQUXXASLVQQ-UHFFFAOYSA-N |
| Inchl | InChI=1S/C19H18O3/c1-2-3-7-17-18(15-6-4-5-8-16(15)22-17)19(21)13-9-11-14(20)12-10-13/h4-6,8-12,20H,2-3,7H2,1H3 |
| IUPAC | (2-butyl-1-benzofuran-3-yl)-(4-hydroxyphenyl)methanone |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Amiodarone HCl is a widely used anti-arrhythmic drug that is used to treat various heart conditions, such as atrial fibrillation, ventricular tachycardia, and ventricular fibrillation. However, during the manufacturing process of amiodarone HCl, impurities may be formed, including Impurity E.
Amidodarone HCl Impurity E is a known impurity that can be formed during the synthesis of amiodarone HCl. This impurity is a potentially harmful substance that can affect the safety and efficacy of the final product. Hence, it is essential to monitor and control the levels of Impurity E during the manufacturing process of amiodarone HCl.
The chemical information of Amidodarone HCl Impurity E is not readily available in the public domain. However, it is known that this impurity is a by-product of the synthesis of amiodarone HCl and may contain similar chemical properties as the parent compound.
In conclusion, Amidodarone HCl Impurity E is a potentially hazardous impurity that can be formed during the synthesis of amiodarone HCl. It is crucial to monitor and control the levels of this impurity during the manufacturing process to ensure the safety and efficacy of the final product.
Get an Instant Quote
Related Compounds
Amiodarone EP Impurity G(HCl salt form) | Des-O-[2-(diethylamino)ethyl]-1-methoxy Amiodarone | Amiodarone EP Impurity G | Amiodarone EP Impurity D | N-Desethyl N-Nitroso Amiodarone | Amiodarone EP Impurity F | Amiodarone EP Impurity C HCl | Des(diethylaminoethyl)-didesiodo-1'-methoxy Amiodarone | N,N-Di-desethyl Amiodarone Hydrochloride | Amiodarone EP Impurity H | Amiodarone Methoxy Impurity | Amiodarone EP Impurity A | Amiodarone EP Impurity C |