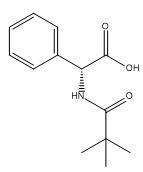
Ampicillin EP Impurity K
| Product Name | Ampicillin EP Impurity K |
|---|---|
| Alternate Names | Ampicillin Impurities, Impurities of Ampicillin |
| CAT No. | CS-O-07209 |
| CAS No. | 40610-41-1 |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | 235.28 g/mol |
| Mol. For. | C₁₃H₁₇NO₃ |
| Hazardous | This is a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Ampicillin |
| Therapeutic | Antibiotics |
| Smileys | OC([C@H](NC(C(C)(C)C)=O)C1=CC=CC=C1)=O |
| Canonical Smiles | CC(C)(C)C(=O)NC(C1=CC=CC=C1)C(=O)O |
| InchIKey | UYMYKMRRCXEIRJ-SNVBAGLBSA-N |
| Inchl | InChI=1S/C13H17NO3/c1-13(2,3)12(17)14-10(11(15)16)9-7-5-4-6-8-9/h4-8,10H,1-3H3,(H,14,17)(H,15,16)/t10-/m1/s1 |
| IUPAC | (2R)-2-(2,2-dimethylpropanoylamino)-2-phenylacetic acid |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Ampicillin EP Impurity K is a chemical compound that is commonly used in the pharmaceutical industry as a reference standard for analytical testing purposes. It is a known impurity of the antibiotic drug Ampicillin, which is used to treat bacterial infections.
Chemically, Ampicillin EP Impurity K is identified as 2,3-dichloro-5,6-dicyano-p-benzoquinone (DDQ) and is a yellowish crystalline powder. DDQ is a strong oxidizing agent that reacts with various organic compounds to form a wide range of products.
In the pharmaceutical industry, Ampicillin EP Impurity K is used as a reference standard for quality control purposes. It is used to ensure that the Ampicillin drug being produced is of high purity and meets the strict regulations set by the Food and Drug Administration (FDA).
Ampicillin EP Impurity K is also used in the development and validation of analytical methods used in the testing of pharmaceuticals. This impurity is used as a marker to identify and quantify the amount of Ampicillin in a sample.
In conclusion, Ampicillin EP Impurity K is an important reference standard used in the pharmaceutical industry. It is used to ensure that the Ampicillin drug being produced is of high quality and meets regulatory standards.
Get an Instant Quote
Related Compounds
Ampicillin thiazepine analog | Ampicillin Dimer Impurity | Ampicillin EP Impurity D | Ampicillin EP Impurity J | Ampicillin oligomer 2 | Ampicillin open ring DImer | Ampicillin EP Impurity I | D-Phenylglycinamide Hydrochloride | Ampicillin EP Impurity E | Ampicillin EP Impurity N | Pivampicillin Impurity B | Ampicillin EP impurity C | Ampicillin EP Impurity L | Ampicillin Oligomer 1 (trimer) Sodium Salt | Ampicillin EP Impurity F | Ampicillin EP Impurity G | Ampicillin EP Impurity I HCl | Ampicillin Impurity p | Ampicillin oligomer 1 (trimer) | Ampicillin EP Impurity B | Pivampicillin Impurity A | Ampicillin Impurity O | N-Piperacillinyl Ampicillin |