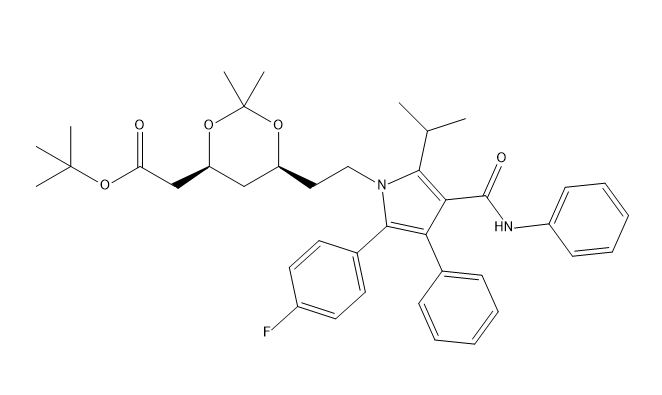
Atorvastatin EP Impurity I
| Product Name | Atorvastatin EP Impurity I |
|---|---|
| Alternate Names | Atorvastatin Impurities, Impurities of Atorvastatin |
| CAT No. | CS-O-07293 |
| CAS No. | 125971-95-1 |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | 654.81 g/mol |
| Mol. For. | C₄₀H₄₇FN₂O₅ |
| Hazardous | This is a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Atorvastatin |
| Purity | 95% |
| Therapeutic | Anti-Diabetic |
| Smileys | O=C(C(C(C1=CC=CC=C1)=C(C2=CC=C(F)C=C2)N3CC[C@@H](OC(C)(C)O4)C[C@@H]4CC(OC(C)(C)C)=O)=C3C(C)C)NC5=CC=CC=C5 |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Atorvastatin EP Impurity I is a chemical compound that is used as a reference standard for the identification and quantification of impurities in Atorvastatin, a commonly prescribed medication for the treatment of high cholesterol. Atorvastatin EP Impurity I is also known as N-(2,2-dimethyl-1-oxopropoxy)-3,5-dihydroxybenzamide and has a molecular formula of C13H17NO5.
The chemical structure of Atorvastatin EP Impurity I is composed of a benzamide moiety substituted with hydroxyl and carboxyl groups. This impurity is one of the major by-products formed during the synthesis of Atorvastatin and its identification is important to ensure the purity of the final drug product.
Atorvastatin EP Impurity I is typically used in analytical laboratories to develop and validate analytical methods for the determination of impurities in Atorvastatin. It can also be used as a reference standard for the purification and isolation of impurities in Atorvastatin.
The chemical properties of Atorvastatin EP Impurity I are well documented in the scientific literature. It has a melting point range of 160-165°C and is sparingly soluble in water. Atorvastatin EP Impurity I is stable under normal storage conditions and does not pose any significant health or safety hazards.
Get an Instant Quote
Related Compounds
Atorvastatin Impurity D | Atorvastatin 2-Fluoro Analog | Atorvastatin Pyrrolidone Phenanthrene Calcium | Atorvastatin 2-Fluoro t-Butyl Ester | Atorvastatin Di-acetonide tert-Butyl Ester | Atorvastatin Diepoxide Impurity | Atorvastatin EP Impurity C Sodium salt | Atorvastatin Cyclic (Fluorophenyl) Impurity | Atorvastatin Oxirane Impurity | Atorvastatin Pyrrolidone Analog | Dihydroxy Diketo Atorvastatin Impurity | Atorvastatin Impurity 25 | sodium (3R,5R)-7-(2-(4-fluorophenyl)-5-isopropyl-3-phenyl-1H-pyrrol-1-yl)-3,5-dihydroxyheptanoate | Atorvastatin EP Impurity C | Atorvastatin Allyl Ester | Atorvastatin EP Impurity B Calcium salt | Atorvastatin EP Impurity E Sodium salt | Atorvastatin 3-Deoxyhept-2E-Enoic Acid | Atorvastatin Impurity 16 | Atorvastatin 2-Hydroxy Sodium | Atorvastatin EP Impurity F (Ca Salt) | Atorvastatin Impurity 17 | Atorvastatin Diketoene Impurity | Atorvastatin EP Impurity D | Atorvastatin Acid t-Butyl Ester | Atorvastatin Cyclic (Fluorophenyl) Sodium | Atorvastatin Lactone 3-O-Methyl Ether | Difluoro Atorvastatin | Atorvastatin Calcium Propylene Glycol Solvate | Atorvastatin EP Impurity F Calcium salt | Atorvastatin EP Impurity P calcium Salt | Atorvastatin Triamino impurity | Atorvastatin Lactam Phenanthrene | Atorvastatin Acid Sodium salt | Atorvastatin 3-Deoxyhept-2Z-Enoic Acid Sodium Salt | Atorvastatin Dehydro Sodium Salt (E/Z mixture) | Atorvastatin Tri-acetonide tert-Butyl Ester | Atorvastatin Cyclic (Fluorophenyl) Impurity | Atorvastatin Impurity 19 | Atorvastatin Lactam Calcium Salt Impurity | Atorvastatin 5-O-Methyl Sodium | Atorvastatin epoxy pyrrolooxazin 7-hydroxy analog | Atorvastatin Impurity F | Allyl Ester of Atorvastatin Cyclic (Fluorophenyl) Impurity | Atorvastatin Dehydro Acid Sodium Salt | Atorvastatin EP Impurity A (Sodium Salt) | Atorvastatin EP Impurity G Sodium Salt | Atorvastatin Epoxy Tetrahydrofuran Analog | Atorvastatin diacid impurity | Atorvastatin Diepoxide Lactone | Atorvastatin Cyclic Fluorophenyl calcium Salt Impurity | Difluoro Atorvastatin Acetonide tert-Butyl Ester | Atorvastatin Lactone Diepoxide | Atorvastatin Impurity 20 | Atorvastatin Diepoxide Calcium Salt | Atorvastatin Ethyl Ester | Atorvastatin Epoxy Pyrrolooxazin Analog | Atorvastatin EP Impurity Q sodium salt | Atorvastatin EP Impurity B | Atorvastatin EP Impurity A (Sodium salt) | N-Nitroso Atorvastatin Impurity | Atorvastatin-ATN-2 triamino impurity | Atorvastatin Isopropyl Ester | ATV-XI | Atorvastatin Diamino impurity | Atorvastatin EP Impurity G (3-O-methyl Atorvastatin calcium salt) | Allyl Ester of Atorvastatin Cyclic (Isopropyl) Impurity | Atorvastatin Impurity 1 | Atorvastatin Epoxy Pyrrolooxazin tricyclic Calcium Salt | Atorvastatin 3-Deoxy-Hept-2-Enoic Acid Ethyl Ester |