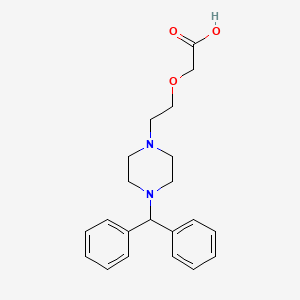
Cetirizine EP Impurity F
| Product Name | Cetirizine EP Impurity F |
|---|---|
| Alternate Names | Cetirizine Impurities, Impurities of Cetirizine |
| CAT No. | CS-O-07604 |
| CAS No. | 83881-53-2 |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | 354.44 g/mol |
| Mol. For. | C₂₁H₂₆N₂O₃ |
| Hazardous | This is a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Cetirizine |
| Purity | Not less than 95 % |
| Therapeutic | Antihistamine |
| Smileys | O=C(O)COCCN1CCN(C(C2=CC=CC=C2)C3=CC=CC=C3)CC1 |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Cetirizine EP Impurity F, also known as (RS)-ethyl 4-(4-chlorophenyl)-6,7-dihydro-1H-imidazo[4,5-c]pyridine-7-carboxylate, is a chemical compound that is commonly used as a reference standard in the pharmaceutical industry. This impurity is an important analytical tool for the identification and quantification of cetirizine, which is an antihistamine drug used to treat allergic rhinitis, hives, and other related conditions.
The usage of Cetirizine EP Impurity F is primarily in the quality control processes of the pharmaceutical industry. It is used to ensure the purity and quality of cetirizine products by comparing the impurity profile of the product with that of the reference standard. This helps to ensure that cetirizine products are safe and effective for use by patients.
In terms of chemical information, Cetirizine EP Impurity F has a molecular formula of C18H15ClN2O2 and a molecular weight of 332.77 g/mol. It is a white to off-white crystalline powder that is sparingly soluble in water and slightly soluble in ethanol, methanol, and acetone. The compound is stable under normal storage conditions and is not known to pose any significant hazards to human health or the environment.
In summary, Cetirizine EP Impurity F is an important reference standard used in the pharmaceutical industry for the quality control of cetirizine products. Its chemical properties and stability make it a safe and effective analytical tool.
Get an Instant Quote
Related Compounds
Cetirizine Polyethylene Glycol (PEG) Ester | Cetirizine Dimer dihydrochloride | Cetirizine Impurity C Dihydrochloride | Cetirizine Glycerol Ester Impurity Hydrochloride | Deschloro Cetirizine Dihydrochloride | Cetirizine EP Impurity E Di Hydrochloride | Cetirizine Impurity 7 | Cetirizine EP Impurity D Dihydrochloride | Cetirizine Impurity 15 DiHCl | Cetirizine USP Related Compound A | Cetirizine Impurity G | Cetirizine Impurity G | Cetirizine Amide Dihydrochloride | Cetirizine USP Related Compund A (2HCl salt) | Cetirizine EP impurity B | Cetirizine Amide | Cetirizine Impurity BHT | Cetirizine EP Impurity C | Cetirizine Impurity BHA | Cetirizine EP Impurity B Ethyl Ester | Cetirizine EP Impurity E | Cetirizine Lactose Ester(Technical Grade) (α/β-mixture, mixture of diastereomers) | Cetirizine Methyl Ester Dihydrochloride | Cetirizine S-Isomer | N-Tosyl Cetirizine EP impurity A | Levocetirizine Impurity 1 | Cetirizine EP impurity A | Levocetirizine Ethyl ester impurity | Cetirizine 3-Chloro | Cetirizine impurity B di Hydrochloride | Cetirizine Lactose Ester HCl salt | Cetirizine 3-Chloro Impurity | Cetirizine S-Isomer di HCl | Levocetirizine benzyl | Cetirizine Glycerol Ester | Cetirizine 1-[(4-Chlorophenyl)phenylmethyl]piperazine Amide | Levocetirizine Lactose ester | Cetirizine Sorbitol Ester Impurity Hydrochloride | Cetirizine Impurity E Sodium Salt | Cetirizine Impurity D | tert-Butyl Cetirizine |