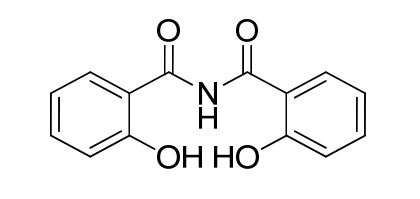
Deferasirox Diacyl Impurity
| Product Name | Deferasirox Diacyl Impurity |
|---|---|
| Alternate Names | Deferasirox Impurities, Impurities of Deferasirox |
| CAT No. | CS-O-07767 |
| CAS No. | 1972-71-0 |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | 257.2 g/mol |
| Mol. For. | C14H11NO4 |
| Hazardous | This is a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Deferasirox |
| Purity | 95% |
| Smileys | O=C(C(C=CC=C1)=C1O)NC(C(C=CC=C2)=C2O)=O |
| Canonical Smiles | C1=CC=C(C(=C1)C(=O)NC(=O)C2=CC=CC=C2O)O |
| InchIKey | QBPYSJJNOYHIPX-UHFFFAOYSA-N |
| Inchl | InChI=1S/C14H11NO4/c16-11-7-3-1-5-9(11)13(18)15-14(19)10-6-2-4-8-12(10)17/h1-8,16-17H,(H,15,18,19) |
| IUPAC | 2-hydroxy-N-(2-hydroxybenzoyl)benzamide |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Deferasirox Diacyl Impurity is a chemical compound that is commonly used in pharmaceutical research and development. It is a byproduct of the synthesis of Deferasirox, which is a medication used to treat iron overload caused by blood transfusions in patients with certain blood disorders.
The chemical structure of Deferasirox Diacyl Impurity is characterized by the presence of two acyl groups, which are organic functional groups containing a carbonyl group bonded to an alkyl or aryl group. The impurity is formed due to the incomplete removal of these acyl groups during the synthesis of Deferasirox.
The usage of Deferasirox Diacyl Impurity is primarily in the laboratory setting for analytical purposes. It is used as a reference material to identify and quantify the impurity in Deferasirox samples. This is important for quality control and assurance as the presence of impurities in pharmaceuticals can affect the safety and effectiveness of the medication.
In terms of chemical properties, Deferasirox Diacyl Impurity is a white to off-white crystalline powder with a melting point of approximately 80-90°C. It is sparingly soluble in water but is soluble in organic solvents such as ethanol and methanol. The compound is stable under normal conditions but may degrade under certain environmental factors such as exposure to light and moisture.
Overall, Deferasirox Diacyl Impurity is an important analytical tool in pharmaceutical research and development. Its chemical properties and usage make it an essential component in ensuring the quality and safety of Deferasirox and other medications.
Get an Instant Quote
Related Compounds
Deferasirox Impurity F | Deferasirox Amide Impurity | Deferasirox Impurity 25 | Deferasirox Phase 1 impurity | Deferasirox Impurity 4 | Deferasirox bis salicylamide impurity | Deferasirox Salicyloyl Ester | Deferasirox Hydrazino Impurity | Deferasirox Isopropyl Ester | Deferasirox Impurity 3 | Azobenzene-4,4'-dicarboxylic Acid | Deferasirox Impurity 22 | Deferasirox Impurity 9 | Deferasirox Impurity 3 | Deferasirox Impurity D | Deferasirox Ethyl Ester |