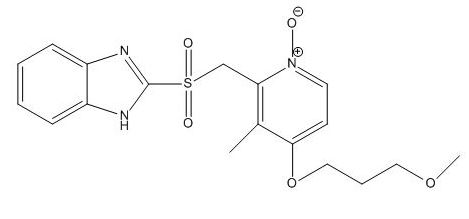
Rabeprazole EP Impurity I
| Product Name | Rabeprazole EP Impurity I |
|---|---|
| Alternate Names | Rabeprazole Impurities, Impurities of Rabeprazole |
| CAT No. | CS-O-08048 |
| CAS No. | 924663-37-6 |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | 391.44 g/mol |
| Mol. For. | C₁₈H₂₁N₃O₅S |
| Hazardous | This is a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Rabeprazole |
| Purity | 95% |
| Therapeutic | Anti ulcer |
| Smileys | O=[S](CC([N](=O)=CC=C1OCCCOC)=C1C)(C2=NC3=CC=CC=C3N2)=O |
| Canonical Smiles | CC1=C(C=C[N+](=C1CS(=O)(=O)C2=NC3=CC=CC=C3N2)[O-])OCCCOC |
| InchIKey | FZBHTBNDQGWAAS-UHFFFAOYSA-N |
| Inchl | InChI=1S/C18H21N3O5S/c1-13-16(21(22)9-8-17(13)26-11-5-10-25-2)12-27(23,24)18-19-14-6-3-4-7-15(14)20-18/h3-4,6-9H,5,10-12H2,1-2H3,(H,19,20) |
| IUPAC | 2-[[4-(3-methoxypropoxy)-3-methyl-1-oxidopyridin-1-ium-2-yl]methylsulfonyl]-1H-benzimidazole |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Rabeprazole EP Impurity I is a chemical compound that is used in the pharmaceutical industry as a reference standard for the analysis of rabeprazole, which is a proton pump inhibitor drug used for the treatment of gastroesophageal reflux disease, ulcers, and other gastrointestinal disorders. The impurity is also known as 1-(3-methoxypropoxy)-3-methylbenzene, and is a derivative of rabeprazole.
The chemical structure of Rabeprazole EP Impurity I includes a benzene ring, a methyl group, and a methoxypropoxy group. It has a molecular formula of C12H18O2 and a molecular weight of 194.27 g/mol. The compound is a white solid that is soluble in organic solvents such as methanol, acetonitrile, and dichloromethane.
In pharmaceutical analysis, Rabeprazole EP Impurity I is used as a standard for the identification and quantification of rabeprazole impurities in drug formulations. It is also used in the development and validation of analytical methods for the determination of rabeprazole in biological fluids such as plasma and urine.
Overall, Rabeprazole EP Impurity I plays a crucial role in ensuring the quality and safety of rabeprazole-containing pharmaceutical products. Its availability as a reference standard enables accurate and reliable analysis of rabeprazole impurities, which is important for the effective treatment of gastrointestinal disorders.
Get an Instant Quote
Related Compounds
Rabeprazole Impurity F | Rabeprazole 5 -chloro Impurity | Rabeprazole Carboxylic Acid Impurity | Rabeprazole 2-Chloromethyl Impurity | Rabeprazole Impurity G Sulfone | Rabeprazole Impurity G | Rabeprazole USP Related Compound A | Rabeprazole Impurity D Sulfide | Rabeprazole 6-chloro Sodium salt | Rabeprazole picolinium salt | Rabeprazole Impurity A | Nitroso Rabeprazole | Rabeprazole Sodium hydrate | Rabeprazole Impurity (Chloro Intermediate) | Rabeprazole Sodium Hydrate | Rabeprazole Impurity 11 | Rabeprazole N-alkylated Sulphide Impurity | Rabeprazole EP Impurity C |