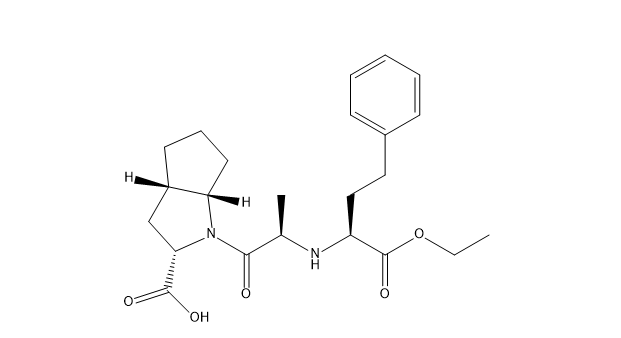
Ramipril EP Impurity I
| Product Name | Ramipril EP Impurity I |
|---|---|
| Alternate Names | Ramipril Impurities, Impurities of Ramipril |
| CAT No. | CS-O-08076 |
| CAS No. | 129939-65-7 |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | 416.51 g/mol |
| Mol. For. | C₂₃H₃₂N₂O₅ |
| Hazardous | This is a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Ramipril |
| Therapeutic | Anti-Hypertensives |
| Smileys | O=C(N1[C@](CCC2)([H])[C@]2([H])C[C@H]1C(O)=O)[C@@H](C)N[C@H](C(OCC)=O)CCC3=CC=CC=C3 |
| Canonical Smiles | CCOC(=O)C(CCC1=CC=CC=C1)NC(C)C(=O)N2C3CCCC3CC2C(=O)O |
| InchIKey | HDACQVRGBOVJII-HTDHLNIYSA-N |
| Inchl | InChI=1S/C23H32N2O5/c1-3-30-23(29)18(13-12-16-8-5-4-6-9-16)24-15(2)21(26)25-19-11-7-10-17(19)14-20(25)22(27)28/h4-6,8-9,15,17-20,24H,3,7,10-14H2,1-2H3,(H,27,28)/t15-,17+,18+,19+,20+/m1/s1 |
| IUPAC | (2S,3aS,6aS)-1-[(2R)-2-[[(2S)-1-ethoxy-1-oxo-4-phenylbutan-2-yl]amino]propanoyl]-3,3a,4,5,6,6a-hexahydro-2H-cyclopenta[b]pyrrole-2-carboxylic acid |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Ramipril EP Impurity I is a pharmaceutical impurity that is used in the analysis and detection of ramipril, which is an angiotensin-converting enzyme inhibitor used in the treatment of hypertension and congestive heart failure. The impurity is a known component of the synthesis of ramipril and is typically found in small quantities in the final product.
Chemically, Ramipril EP Impurity I is a dipeptide derivative that contains the amino acid proline in its structure. It has a molecular weight of 318.4 g/mol and a chemical formula of C13H18N2O3. The impurity is typically identified and quantified using high-performance liquid chromatography (HPLC) and is often used as a reference standard in the analysis of ramipril.
The usage of Ramipril EP Impurity I is important in ensuring the quality and purity of ramipril. Impurities in pharmaceuticals can affect the efficacy and safety of the drug, which is why regulatory agencies require strict control over impurities in pharmaceutical products. By using Ramipril EP Impurity I as a reference standard, pharmaceutical companies can ensure that their ramipril products meet the required standards for purity and quality.
In conclusion, Ramipril EP Impurity I is an important reference standard in the analysis and detection of ramipril, a commonly used medication for hypertension and congestive heart failure. Its chemical properties and usage in quality control ensure that ramipril products are safe and effective for patients.
Get an Instant Quote
Related Compounds
Cyclohexyl Ramipril Hydrochloride | Ramipril EP Impurity J | Ramipril EP Impurity A | Ramipril - Isomer Impurity 1 | Ramipril EP Impurity K | Tris(hydroxymethyl) aminomethane salt of ramipril 2 | RAMIPRIL IMPURITY K DIASTEREOMER I | N-Nitroso-Ramipril in 1mg/1ml in methanol | N-Nitroso Ramipril (Mixture of isomers) | ramipril keto impurity | Ramipril EP Impurity G | Ramipril EP Impurity M | Ramipril EP Impurity D | ent-Ramipril | Ramipril isopropyl ester | Ramipril EP Impurity H | Ramipril EP Impurity C | Tris(hydroxymethyl) aminomethane salt of ramipril 1 | Ramipril Benzyl Ester | Ramipril EP Impurity O |