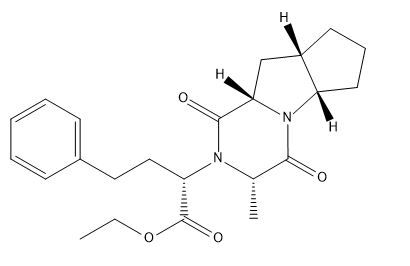
Ramipril EP Impurity D
| Product Name | Ramipril EP Impurity D |
|---|---|
| Alternate Names | Ramipril Impurities, Impurities of Ramipril |
| CAT No. | CS-O-08082 |
| CAS No. | 108731-95-9 |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | 398.50 g/mol |
| Mol. For. | C₂₃H₃₀N₂O₄ |
| Hazardous | This is a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Ramipril |
| Purity | >98% |
| Therapeutic | Anti-Hypertensives |
| Smileys | O=C([C@@H](N1[C@H](C(OCC)=O)CCC2=CC=CC=C2)C)N([C@](CCC3)([H])[C@]3([H])C4)[C@]4([H])C1=O |
| Canonical Smiles | CCOC(=O)C(CCC1=CC=CC=C1)N2C(C(=O)N3C4CCCC4CC3C2=O)C |
| InchIKey | KOVMAAYRBJCASY-JBDAPHQKSA-N |
| Inchl | InChI=1S/C23H30N2O4/c1-3-29-23(28)19(13-12-16-8-5-4-6-9-16)24-15(2)21(26)25-18-11-7-10-17(18)14-20(25)22(24)27/h4-6,8-9,15,17-20H,3,7,10-14H2,1-2H3/t15-,17-,18-,19-,20-/m0/s1 |
| IUPAC | ethyl (2S)-2-[(2S,6S,8S,11S)-11-methyl-9,12-dioxo-1,10-diazatricyclo[6.4.0.02,6]dodecan-10-yl]-4-phenylbutanoate |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Ramipril EP Impurity D is a chemical compound that is used in the pharmaceutical industry as a reference standard and impurity marker for the drug Ramipril. Ramipril is a widely prescribed medication used to treat high blood pressure and heart failure. Ramipril EP Impurity D is used as a quality control standard to ensure the purity and potency of Ramipril in drug formulations.
Chemically, Ramipril EP Impurity D is known as (2S,3S)-3-[(2S)-2-[(1S)-1-ethoxy-1-oxo-4-phenylbutan-2-yl] pyrrolidin-1-yl]-2-methyl-4-oxo-pentanoic acid. The compound has a molecular weight of 461.59 g/mol and a melting point of 165-170°C. It is a white or off-white powder that is soluble in polar solvents such as methanol and water.
Ramipril EP Impurity D is synthesized through a series of chemical reactions starting from commercially available starting materials. The compound is purified through various chromatographic techniques to achieve the desired purity level. The purity of the compound is determined through analytical techniques such as high-performance liquid chromatography (HPLC) and mass spectrometry (MS).
Overall, Ramipril EP Impurity D plays an important role in the quality control of Ramipril drug formulations. Its availability as a reference standard ensures the accuracy and reliability of analytical methods used to test Ramipril drug products.
Get an Instant Quote
Related Compounds
Tris(hydroxymethyl) aminomethane salt of ramipril 1 | N-Nitroso Ramipril (Mixture of isomers) | RAMIPRIL IMPURITY K DIASTEREOMER I | Ramipril EP Impurity H | ramipril keto impurity | Ramipril Benzyl Ester | Ramipril EP Impurity A | Cyclohexyl Ramipril Hydrochloride | Ramipril isopropyl ester | Ramipril - Isomer Impurity 1 | ent-Ramipril | Ramipril EP Impurity O | Ramipril EP Impurity I | N-Nitroso-Ramipril in 1mg/1ml in methanol | Tris(hydroxymethyl) aminomethane salt of ramipril 2 | Ramipril EP Impurity G | Ramipril EP Impurity C | Ramipril EP Impurity K | Ramipril EP Impurity J | Ramipril EP Impurity M |