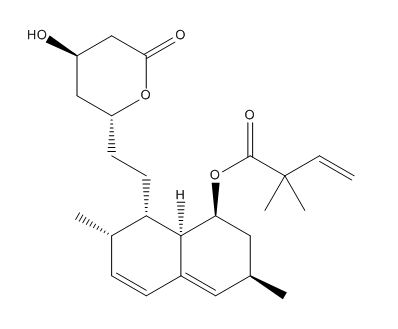
Simvastatin EP Impurity G
| Product Name | Simvastatin EP Impurity G |
|---|---|
| Alternate Names | Simvastatin Impurities, Impurities of Simvastatin |
| CAT No. | CS-O-08185 |
| CAS No. | 79902-62-8 |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | 416.55 g/mol |
| Mol. For. | C₂₅H₃₆O₅ |
| Hazardous | This is a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Simvastatin |
| Purity | 95% |
| Therapeutic | Anti-Diabetic |
| Smileys | CCC(C)(C)C(=O)OC1CC(C=C2C1C(C(C=C2)C)CCC3CC(CC(=O)O3)O)C |
| Canonical Smiles | CCC(C)(C)C(=O)OC1CC(C=C2C1C(C(C=C2)C)CCC3CC(CC(=O)O3)O)C |
| InchIKey | RYMZZMVNJRMUDD-MKPQAFFTSA-N |
| Inchl | InChI=1S/C25H38O5/c1-6-25(4,5)24(28)30-21-12-15(2)11-17-8-7-16(3)20(23(17)21)10-9-19-13-18(26)14-22(27)29-19/h7-8,11,15-16,18-21,23,26H,6,9-10,12-14H2,1-5H3/t15-,16-,18+,19-,20-,21-,23-/m1/s1 |
| IUPAC | [(1R,3S,7R,8R,8aS)-8-[2-[(2R,4S)-4-hydroxy-6-oxooxan-2-yl]ethyl]-3,7-dimethyl-1,2,3,7,8,8a-hexahydronaphthalen-1-yl] 2,2-dimethylbutanoate |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Simvastatin EP Impurity G is a chemical compound that is commonly used in the pharmaceutical industry. It is a by-product of the synthesis of Simvastatin, which is a cholesterol-lowering drug. Simvastatin EP Impurity G is formed during the manufacturing process of Simvastatin and is usually present in low concentrations.
The usage of Simvastatin EP Impurity G is to ascertain the purity of Simvastatin. It is used as a reference standard in quality control testing. This impurity is identified and quantified using analytical techniques such as high-performance liquid chromatography (HPLC). The presence of Simvastatin EP Impurity G in Simvastatin can affect the efficacy and safety of the drug, therefore it is imperative to monitor its levels.
From a chemical perspective, Simvastatin EP Impurity G is a dihydroxy acid lactone derivative. Its molecular formula is C26H42O5 and its molecular weight is 442.61 g/mol. This impurity has a melting point of approximately 148-150°C and is sparingly soluble in water.
In conclusion, Simvastatin EP Impurity G is an important reference standard in the quality control testing of Simvastatin. Its chemical properties and low solubility in water make it an ideal candidate for analytical testing.
Get an Instant Quote
Related Compounds
Simvastatin Impurity K | Simvastatin 4'-Methyl Ether | Simvastatin (6S)-Hydroxy Isomer | Simvastatin Anhydro Acid Sodium Salt | Simvastatin EP Impurity K | Simvastatin pivaloyl impurity | Simvastatin EP Impurity E | Simvastatin EP Impurity D | Simvastatin Acid Methyl Ester | Simvastatin Acid Glycerol Ester | Simvastatin (3R)-Hydroxy Impurity | Simvastatin EP Impurity C | Simvastatin (6R)-Hydroxy Isomer | Simvastatin 6-Oxo Isomer | Simvastatin (3S)-Hydroxy Impurity | Simvastatin EP Impurity B | Simvastatin EP Impurity A | Simvastatin pivaloyl Dehydro impurity | Simvastatin Acid Ethyl Ester | Simvastatin, 1-Pyreneacetyl Ester |