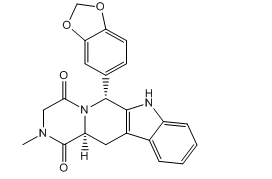
Tadalafil EP Impurity A
| Product Name | Tadalafil EP Impurity A |
|---|---|
| Alternate Names | Tadalafil Impurities, Impurities of Tadalafil |
| CAT No. | CS-O-08227 |
| CAS No. | 171596-27-3 |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | 389.4 g/mol |
| Mol. For. | C22H19N3O4 |
| Hazardous | This is a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Tadalafil |
| Purity | 95% |
| Therapeutic | Erectile dysfunction |
| Smileys | O=C(N1[C@@H](C2=C(C[C@]1(C3=O)[H])C4=CC=CC=C4N2)C5=CC6=C(C=C5)OCO6)CN3C |
| Canonical Smiles | CN1CC(=O)N2C(C1=O)CC3=C(C2C4=CC5=C(C=C4)OCO5)NC6=CC=CC=C36 |
| InchIKey | WOXKDUGGOYFFRN-HRAATJIYSA-N |
| Inchl | InChI=1S/C22H19N3O4/c1-24-10-19(26)25-16(22(24)27)9-14-13-4-2-3-5-15(13)23-20(14)21(25)12-6-7-17-18(8-12)29-11-28-17/h2-8,16,21,23H,9-11H2,1H3/t16-,21+/m0/s1 |
| IUPAC | (2R,8S)-2-(1,3-benzodioxol-5-yl)-6-methyl-3,6,17-triazatetracyclo[8.7.0.03,8.011,16]heptadeca-1(10),11,13,15-tetraene-4,7-dione |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Tadalafil EP Impurity A is a chemical substance that is commonly used in the pharmaceutical industry for the development of drugs that treat erectile dysfunction, pulmonary arterial hypertension, and other similar medical conditions. This impurity is often used as a reference standard in the quality control of tadalafil formulations.
Tadalafil EP Impurity A is a highly purified substance that is obtained through various purification processes, including chromatography and crystallization. It is a white to off-white crystalline powder that has a molecular formula of C22H19N3O4 and a molecular weight of 393.4 g/mol.
Chemically, Tadalafil EP Impurity A is a structural isomer of tadalafil, which is a phosphodiesterase type 5 (PDE5) inhibitor. This impurity shares the same core structure as tadalafil, but with subtle differences in the arrangement of its functional groups.
When used as a reference standard, Tadalafil EP Impurity A is typically analyzed using various analytical techniques, such as high-performance liquid chromatography (HPLC), gas chromatography (GC), and nuclear magnetic resonance (NMR) spectroscopy. These techniques are used to confirm the identity and purity of the impurity, as well as to ensure that it meets the necessary quality standards for use in pharmaceutical formulations.
Overall, Tadalafil EP Impurity A is a vital component in the development and quality control of tadalafil-based drugs, and its chemical properties and usage are critical for ensuring the safety and efficacy of these medications.
Get an Instant Quote
Related Compounds
Tadalafil Impurity D (S,S)Isomer | Tadalafil EP Impurity C | Tadalafil Ketolactam | Tadalafil Related Compound 2 | Tadalafil impurity F | Tadalafil Impurity 7 | N-Desmethyl Tadalafil | Tadalafil chloroacetyl Impurity | Tadalafil impurity E | N-Ethyl tadalafil | Tadalafil EP Impurity I | Tadalafil Impurity 13 | Tadalafil Acid Impurity (HCl) | N-Nitroso Chloropretadalafil | Tadalafil EP Impurity D | Tadalafil Impurity 5 | Tadalafil Impurity 4 | Tadalafil impurity G | Tadalafil EP Impurity B | Amino Tadalafil | Tadalafil EP Impurity I | Tadalafil Impurity 3 | Tadalafil Impurity 4 | Chloropropanoylpretadalafil | Tadalafil EP Impurity C hydrochloride trihydrate | Chloropretadalafil (S,S-isomer) | Tadalafil Nitroso Impurity 1 | Tadalafil EP Impurity I (Hydrate) | Tadalafil Impurity H | Tadalafil Impurity 6 | Tadalafil Impurity 10 | Tadalafil Hydroxypiperidone | N-nitroso Tadalafil | Tadalafil Impurity 14 | Tadalafil Impurity 37 | Tadalafil Desmethylene Impurity |