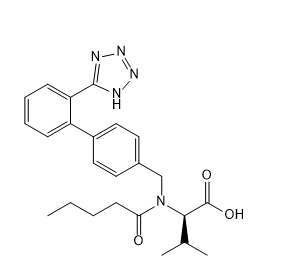
Valsartan EP impurity A
| Product Name | Valsartan EP impurity A |
|---|---|
| Alternate Names | Valsartan Impurities, Impurities of Valsartan |
| CAT No. | CS-O-08393 |
| CAS No. | 137862-87-4 |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | 435.52 g/mol |
| Mol. For. | C₂₄H₂₉N₅O₃ |
| Hazardous | This is a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Valsartan |
| Purity | >98% |
| Therapeutic | Anti-Hypertensives |
| Smileys | CCCCC(=O)N(CC1=CC=C(C=C1)C2=CC=CC=C2C3=NNN=N3)C(C(C)C)C(=O)O |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Valsartan EP Impurity A is a chemical compound that is commonly used in the pharmaceutical industry. It is a known impurity of Valsartan, a medication used to treat high blood pressure and heart failure. Valsartan EP Impurity A has the chemical formula C24H26N2O3S and a molecular weight of 422.54 g/mol. This impurity is known to be a significant byproduct during the manufacturing process of Valsartan.
The presence of Valsartan EP Impurity A in Valsartan medication can have adverse effects on the patient's health. Therefore, the pharmaceutical industry strictly regulates the acceptable levels of Valsartan EP Impurity A in Valsartan medication. It is essential to monitor and control the levels of this impurity to ensure the safety and efficacy of the medication.
The chemical structure of Valsartan EP Impurity A contains a benzimidazole ring, a phenyl group, and a sulfonamide group. These chemical groups are known to have potent biological activities and can cause adverse effects on the human body. Therefore, it is crucial to monitor and control the levels of this impurity in Valsartan medication.
In conclusion, Valsartan EP Impurity A is a significant impurity in Valsartan medication. It is essential to monitor and control its levels to ensure the safety and efficacy of the medication. The pharmaceutical industry must adhere to strict regulations to ensure that the medication is safe for human consumption.
Get an Instant Quote
Related Compounds
Valsartan Nitraso impurity -21 (Mixture of isomers) | Valsartan Impurity 18 | Valsartan Ethyl Ester | Valsartan Dimer Impurity | N-nitroso(tetrazole) Valsartan Desvaleryl Impurity | N-Nitroso Sacubitril Valsartan | N-(1-Oxopropyl)-N-[[2′-(2H-tetrazol-5-yl)[1,1′-biphenyl]-4-yl]methyl]-L-valine | Valsartan L-Luceine | N-Nitroso N-Devaleryl Valsartan methyl ester Impurity (mixture of isomers) | Valsartan Impurity 23 | N-Nitroso Valsartan | rac-Valsartan Ethyl Ester | Valsartan Impurity 28 | Valsartan N-Oxide 2 | Valsartan EP Impurity C | N-nitroso Valsartan Desvaleryl Impurity | Racemic mixture of Valsartan and valsartan related compound A | Trityl valsartan methyl ester | Valsartan Ditetrazole Impurity | Valsartan Azide impurity | Valsartan EP Impurity B | Valsartan Impurity 22 | N,N-dinitroso Valsartan Desvaleryl Impurity | Valsartan Impurity 22 |