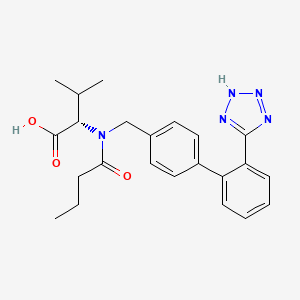
Valsartan EP Impurity C
| Product Name | Valsartan EP Impurity C |
|---|---|
| Alternate Names | Valsartan Impurities, Impurities of Valsartan |
| CAT No. | CS-O-08394 |
| CAS No. | 952652-79-8 |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | 421.49 g/mol |
| Mol. For. | C₂₃H₂₇N₅O₃ |
| Hazardous | This is a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Valsartan |
| Purity | >98% |
| Therapeutic | Anti-Hypertensives |
| Smileys | O=C(CCC)N([C@H](C(O)=O)C(C)C)CC1=CC=C(C2=C(C3=NN=NN3)C=CC=C2)C=C1 |
| Canonical Smiles | CCCC(=O)N(CC1=CC=C(C=C1)C2=CC=CC=C2C3=NNN=N3)C(C(C)C)C(=O)O |
| InchIKey | OKAQHVJSXLGXET-NRFANRHFSA-N |
| Inchl | InChI=1S/C23H27N5O3/c1-4-7-20(29)28(21(15(2)3)23(30)31)14-16-10-12-17(13-11-16)18-8-5-6-9-19(18)22-24-26-27-25-22/h5-6,8-13,15,21H,4,7,14H2,1-3H3,(H,30,31)(H,24,25,26,27)/t21-/m0/s1 |
| IUPAC | (2S)-2-[butanoyl-[[4-[2-(2H-tetrazol-5-yl)phenyl]phenyl]methyl]amino]-3-methylbutanoic acid |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Valsartan EP Impurity C is a chemical compound that is commonly used in the pharmaceutical industry for the production of medications. It is a known impurity of Valsartan, which is a medication used for the treatment of high blood pressure and heart failure. Valsartan EP Impurity C is chemically known as 2-Butyl-4-chloro-5-formylimidazole and is also referred to as Valsartan related compound A.
The chemical structure of Valsartan EP Impurity C comprises of an imidazole ring, a butyl group, a chlorine atom, and a formyl group. The compound is usually used as a reference standard in the quality control of Valsartan drugs. It is used to test the purity of Valsartan and ensure that the medication is free from any impurities that can be harmful to human health.
In terms of usage, Valsartan EP Impurity C is usually used in analytical laboratories to determine the quantity of Valsartan in a given sample. It is also used in research and development to study the properties and behavior of Valsartan drugs. Additionally, it can be used to develop new formulations and dosage forms of Valsartan.
It is important to note that Valsartan EP Impurity C is not intended for human consumption as it is an impurity and may pose health risks when ingested. The compound should be handled with caution and stored in a cool and dry place away from direct sunlight.
Get an Instant Quote
Related Compounds
Valsartan Impurity 22 | N-nitroso(tetrazole) Valsartan Desvaleryl Impurity | Trityl valsartan methyl ester | N-(1-Oxopropyl)-N-[[2′-(2H-tetrazol-5-yl)[1,1′-biphenyl]-4-yl]methyl]-L-valine | N-Nitroso N-Devaleryl Valsartan methyl ester Impurity (mixture of isomers) | Valsartan Nitraso impurity -21 (Mixture of isomers) | N-Nitroso Valsartan | Racemic mixture of Valsartan and valsartan related compound A | Valsartan Ethyl Ester | Valsartan N-Oxide 2 | Valsartan Impurity 22 | N-nitroso Valsartan Desvaleryl Impurity | Valsartan Azide impurity | Valsartan EP impurity A | N,N-dinitroso Valsartan Desvaleryl Impurity | N-Nitroso Sacubitril Valsartan | Valsartan Ditetrazole Impurity | Valsartan Impurity 23 | Valsartan L-Luceine | Valsartan EP Impurity B | rac-Valsartan Ethyl Ester | Valsartan Dimer Impurity | Valsartan Impurity 28 | Valsartan Impurity 18 |