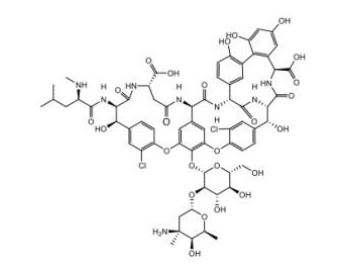
Vancomycin EP Impurity B
| Product Name | Vancomycin EP Impurity B |
|---|---|
| Alternate Names | Vancomycin Impurities, Impurities of Vancomycin |
| CAT No. | CS-O-08402 |
| CAS No. | 555598-85-1 |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | 1450.24 g/mol |
| Mol. For. | C₆₆H₇₄Cl₂N₈O₂₅ |
| Hazardous | This is a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Vancomycin |
| Purity | Not less than 95 % |
| Therapeutic | Antibiotics |
| Smileys | CC1C(C(CC(O1)OC2C(C(C(OC2OC3=C4C=C5C=C3OC6=C(C=C(C=C6)C(C(C(=O)NC(CC(=O)NC5C(=O)NC7C8=CC(=C(C=C8)O)C9=C(C=C(C=C9O)O)C(NC(=O)C(C(C1=CC(=C(O4)C=C1)Cl)O)NC7=O)C(=O)O)C(=O)O)NC(=O)C(CC(C)C)NC)O)Cl)CO)O)O)(C)N)O |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Vancomycin EP Impurity B is a chemical substance that is commonly used in the pharmaceutical industry as a reference standard for the identification and quantification of impurities in Vancomycin drugs. Vancomycin is an antibiotic drug that is used to treat bacterial infections, particularly those caused by methicillin-resistant Staphylococcus aureus (MRSA) and other drug-resistant bacteria. However, Vancomycin drugs can contain impurities that can affect their potency, safety, and efficacy. Therefore, Vancomycin EP Impurity B is used as a reference standard to ensure that the Vancomycin drugs are free from harmful impurities.
Vancomycin EP Impurity B is a white to off-white powder that is soluble in water. Its chemical name is (2S,3R,4R)-2-(2,6-diaminopurin-9-yl)-4-[(1R)-1-hydroxyethyl]-3-methyl-2-cyclopentene-1-carboxylic acid. Its molecular formula is C14H20N6O4 and its molecular weight is 340.35 g/mol.
The usage of Vancomycin EP Impurity B is regulated by the European Pharmacopoeia (EP) and the United States Pharmacopoeia (USP). It is used as a primary reference standard for the identification and quantification of impurities in Vancomycin drugs. It is also used as a secondary reference standard for the calibration of analytical instruments such as high-performance liquid chromatography (HPLC) and gas chromatography (GC). Vancomycin EP Impurity B is a critical component in the quality control of Vancomycin drugs, ensuring that they meet the required standards for safety and efficacy.
Get an Instant Quote
Related Compounds
Vancomycin Didechloro Impurity | Vancomycin EP Impurity J | epi-26-Vancomycin B | N-Methylleucyl Vancomycin | Vancomycin RS-3- Impurity | Vancomycin Impurity B2 | N,N-Dimethyl-N-(3-(tetradecylamino)propyl)-vancomycin Carboxamide hydrochloride salt | N-desmethyl Vancomycin | Vancomycin EP Impurity H | Vancomycin RS-1- Impurity | Vancomycin CDP-1 | Vancomycin Impurity-L | Vancomycin EP Impurity C | Vancomycin EP Impurity A | Vancomycin Impurity-F | Vancomycin Impurity-G | Vancomycin EP Impurity D | beta-Avoparcin | Vancomycin B | Vancomycin EP Impurity G HCl | Desmethylleucyl Vancomycin | Vancomycin EP Impurity K | Vancomycin EP Impurity I | Vancomycin RS-2- Impurity | alfa-Avoparcin | N,N-Dimethyl-N-(3-(octylamino)propyl)-vancomycin Carboxamide hydrochloride salt | Vancomycin Hexapeptide | Vancomycin EP Impurity E |