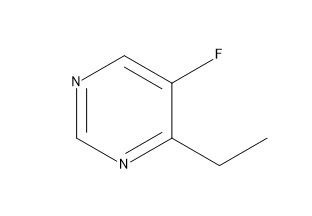
Voriconazole EP Impurity C
| Product Name | Voriconazole EP Impurity C |
|---|---|
| Alternate Names | Voriconazole Impurities, Impurities of Voriconazole |
| CAT No. | CS-O-08456 |
| CAS No. | 137234-88-9 |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | 126.13 g/mol |
| Mol. For. | C₆H₇FN₂ |
| Hazardous | This is a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Voriconazole |
| Canonical Smiles | CCC1=NC=NC=C1F |
| InchIKey | AYZDRTRWCASUFO-UHFFFAOYSA-N |
| Inchl | InChI=1S/C6H7FN2/c1-2-6-5(7)3-8-4-9-6/h3-4H,2H2,1H3 |
| IUPAC | 4-ethyl-5-fluoropyrimidine |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Voriconazole EP Impurity C is a chemical compound that is commonly used in pharmaceutical research and development. This compound is a byproduct of the synthesis of Voriconazole, an antifungal medication used to treat various fungal infections. Voriconazole EP Impurity C can be found in small quantities in Voriconazole, and it is essential to understand the properties and characteristics of this impurity to ensure the safety and efficacy of the final drug product.
The usage of Voriconazole EP Impurity C in pharmaceutical research is primarily focused on understanding its pharmacological properties and potential side effects. Researchers study this compound to identify any potential impurities that may be present in Voriconazole and to ensure that the final drug product is safe for use in humans. Additionally, Voriconazole EP Impurity C is used to study the metabolic pathways of Voriconazole and to identify any potential interactions with other medications.
The chemical information of Voriconazole EP Impurity C is crucial in understanding its pharmacological properties. This compound is a complex organic molecule that contains several functional groups, including an amine and an imidazole ring. It has a molecular weight of 327.4 g/mol and a melting point of approximately 130°C. The chemical properties of Voriconazole EP Impurity C are also essential in determining its stability, solubility, and bioavailability.
In conclusion, Voriconazole EP Impurity C is an essential compound in pharmaceutical research and development, particularly in the development of Voriconazole. The study of this compound is vital in ensuring the safety and efficacy of the final drug product, and understanding its properties and characteristics is crucial in identifying any potential impurities and interactions with other medications.
Get an Instant Quote
Related Compounds
Voriconazole EP Impurity E | Voriconazole USP Related Compound B | Voriconazole Impurity 18 (Mixture of Diastereomers) | Voriconazole Impurity 7 | Voriconazole 6-Chloro Impurity-6-Chloro Voriconazole | Voriconazole EP Impurity B | Chloro voriconazole diastereomer | Voriconazole USP Related Compound A | rel-(R,R)-Voriconazole |