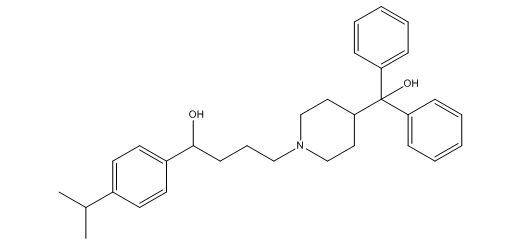
Fexofenadine Hydrochloride EP Impurity C
| Product Name | Fexofenadine Hydrochloride EP Impurity C |
|---|---|
| Alternate Names | Fexofenadine Impurities, Impurities of Fexofenadine |
| CAT No. | CS-O-10070 |
| CAS No. | 185066-37-9 |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | 457.65 g/mol |
| Mol. For. | C₃₁H₃₉NO₂ |
| Hazardous | This is a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Fexofenadine |
| Purity | >98% |
| Therapeutic | Antihistamine |
| Smileys | OC(C1=CC=CC=C1)(C2=CC=CC=C2)C3CCN(CCCC(C4=CC=C(C(C)C)C=C4)O)CC3 |
| Canonical Smiles | CC(C)C1=CC=C(C=C1)C(CCCN2CCC(CC2)C(C3=CC=CC=C3)(C4=CC=CC=C4)O)O |
| InchIKey | FZSQPQGERLSIOR-UHFFFAOYSA-N |
| Inchl | InChI=1S/C31H39NO2/c1-24(2)25-15-17-26(18-16-25)30(33)14-9-21-32-22-19-29(20-23-32)31(34,27-10-5-3-6-11-27)28-12-7-4-8-13-28/h3-8,10-13,15-18,24,29-30,33-34H,9,14,19-23H2,1-2H3 |
| IUPAC | 4-[4-[hydroxy(diphenyl)methyl]piperidin-1-yl]-1-(4-propan-2-ylphenyl)butan-1-ol |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Fexofenadine Hydrochloride EP Impurity C is a chemical compound that is commonly used in the pharmaceutical industry as a reference standard for the analysis and identification of impurities in Fexofenadine Hydrochloride. Fexofenadine Hydrochloride is an antihistamine medication that is used to treat various allergic conditions such as seasonal allergies, hay fever, and hives.
The chemical formula of Fexofenadine Hydrochloride EP Impurity C is C16H18ClNO2, and its molecular weight is 295.77 g/mol. It is a white to off-white colored crystalline powder that is sparingly soluble in water and ethanol.
Fexofenadine Hydrochloride EP Impurity C is typically used in quality control laboratories to ensure the purity and potency of Fexofenadine Hydrochloride. It is also used in research and development to study the pharmacology and toxicology of Fexofenadine Hydrochloride and its related compounds.
The safety and toxicity of Fexofenadine Hydrochloride EP Impurity C are not well established, and caution should be exercised when handling this compound. It should be stored in a cool, dry place away from heat and light sources, and should be handled by trained professionals only.
Get an Instant Quote
Related Compounds
Fexofenadine Hydrochloride EP Impurity F | Fexofenadine Hydrochloride EP Impurity G | Fexofenadine Hydrochloride EP Impurity D | Fexofenadine Impurity 37 | Fexofenadine Tertiary dehydrated Impurity | meta-Fexofenadine Hydrochloride | Fexofenadine Related Compound B | Fexofenadine P-Ethyl Ester | Fexafenadine EP Impurity B | Fexofenadine HCl Impurity L | Fexofenadine hydrochloride USP Impurity A | Fexofenadine Related Compound C | Fexofenadine EP Impurity A | FEXOFENADINE EP IMPURITY A - HCl | Fexofenadine N-Oxide |