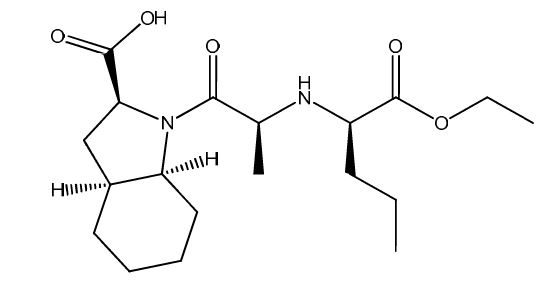
Perindopril EP Impurity I
| Product Name | Perindopril EP Impurity I |
|---|---|
| CAT No. | CS-O-10988 |
| CAS No. | 145513-33-3 |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | 368.47 g/mol |
| Mol. For. | C₁₉H₃₂N₂O₅ |
| Hazardous | This is a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Therapeutic | Anti-Hypertensives |
| Smileys | O=C(N1[C@](CCCC2)([H])[C@]2([H])C[C@H]1C(O)=O)[C@H](C)N[C@H](CCC)C(OCC)=O |
| Canonical Smiles | CCCC(C(=O)OCC)NC(C)C(=O)N1C2CCCCC2CC1C(=O)O |
| InchIKey | IPVQLZZIHOAWMC-QMHWVQJVSA-N |
| Inchl | InChI=1S/C19H32N2O5/c1-4-8-14(19(25)26-5-2)20-12(3)17(22)21-15-10-7-6-9-13(15)11-16(21)18(23)24/h12-16,20H,4-11H2,1-3H3,(H,23,24)/t12-,13-,14+,15-,16-/m0/s1 |
| IUPAC | (2S,3aS,7aS)-1-[(2S)-2-[[(2R)-1-ethoxy-1-oxopentan-2-yl]amino]propanoyl]-2,3,3a,4,5,6,7,7a-octahydroindole-2-carboxylic acid |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Perindopril EP Impurity I is a pharmaceutical impurity that is commonly used in the production of antihypertensive drugs such as Perindopril. The impurity is added during the synthesis process of Perindopril to enhance its therapeutic effects. The chemical name of Perindopril EP Impurity I is (2S,3aS,7aS)-1-[(2S)-2-[[(2S)-2-[(1S)-1-carboxybutyl]amino]propanoyl]amino]octahydro-1H-indole-2-carboxylic acid.
Perindopril EP Impurity I is a chiral compound, which means that it exists in two mirror-image forms known as enantiomers. These enantiomers have different biological activities, and the production of Perindopril involves the separation of the enantiomers to produce the desired therapeutic effect.
The usage and chemical information of Perindopril EP Impurity I are critical to the quality control and regulatory compliance of Perindopril-based drugs. The impurity is closely monitored during the production process to ensure that it meets the strict quality standards required by the pharmaceutical industry. The chemical properties of Perindopril EP Impurity I are also important in determining its stability, solubility, and bioavailability, which affect its therapeutic efficacy and safety.
In conclusion, Perindopril EP Impurity I is an essential component in the production of Perindopril-based antihypertensive drugs. Its usage and chemical information play a crucial role in ensuring the quality and safety of these drugs, making it a vital component of the pharmaceutical industry.
Get an Instant Quote
This page contains information about Perindopril EP Impurity I. You can buy Perindopril EP Impurity I from Clearsynth at best competitive price with assured price guarantee. Clearsynth offers best quality Perindopril EP Impurity I