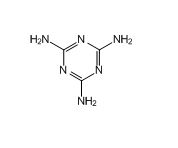
Metformin EP impurity D
| Product Name | Metformin EP impurity D |
|---|---|
| Alternate Names | Metformin Impurities, Impurities of Metformin |
| CAT No. | CS-O-11683 |
| CAS No. | 108-78-1 |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | 126.12 g/mol |
| Mol. For. | C₃H₆N₆ |
| Hazardous | This is a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Metformin |
| Purity | 95% |
| Therapeutic | Anti-Diabetic |
| Smileys | C1(=NC(=NC(=N1)N)N)N |
| Canonical Smiles | C1(=NC(=NC(=N1)N)N)N |
| InchIKey | JDSHMPZPIAZGSV-UHFFFAOYSA-N |
| Inchl | InChI=1S/C3H6N6/c4-1-7-2(5)9-3(6)8-1/h(H6,4,5,6,7,8,9) |
| IUPAC | 1,3,5-triazine-2,4,6-triamine |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Metformin EP impurity D is a residual impurity that can be found in Metformin, which is a commonly used oral anti-diabetic medication. Although Metformin is a highly effective medication, it can sometimes contain impurities such as Metformin EP impurity D.
Metformin EP impurity D is a chemical compound that is classified as a secondary amine. Its chemical name is N-(2-aminoethyl)-N-methylacetamide, and its molecular formula is C5H12N2O. This impurity is known to be a potential carcinogenic compound, which is why its presence in Metformin must be monitored and controlled.
The usage of Metformin EP impurity D is strictly regulated by the European Pharmacopeia, which sets the maximum allowable limit for this impurity. The acceptable limit of Metformin EP impurity D in Metformin is set to less than or equal to 0.1%. Any amount above this limit is considered to be unsafe and can pose a risk to the health of the patient.
To minimize the presence of Metformin EP impurity D in Metformin, manufacturers must adhere to strict quality control measures during the manufacturing process. They must also conduct regular testing and analysis of their products to ensure compliance with regulatory guidelines. Overall, the usage and chemical information of Metformin EP impurity D is important for the safety and efficacy of Metformin as a medication.
Get an Instant Quote
Related Compounds
Metformin Impurity F | N-Nitroso Metformin | Metformin Impurity E | Metformin EP impurity B Hydrochloride | Metformin EP impurity C | Metformin EP impurity B | Metformin - Impurity E (Sulfate Salt) | Metformin EP Impurity B NITRATE salt | Metformin Impurity E sulfate salt | Sitagliptin+ Metformin Imp 4 | Metformin EP impurity F (2M in THF) | Metformin Hydroxy Analog 1 | Metformin EP Impurity A |