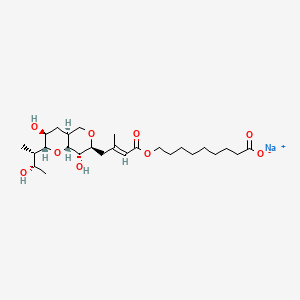
Mupirocin calcium EP impurity E sodium salt
| Product Name | Mupirocin calcium EP impurity E sodium salt |
|---|---|
| Alternate Names | Mupirocin Impurities, Impurities of Mupirocin |
| CAT No. | CS-O-11834 |
| CAS No. | 116182-44-6 |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | 522.6 g/mol |
| Mol. For. | C₂₆H₄₃NaO₉ |
| Hazardous | This is a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Mupirocin |
| Purity | 95% |
| Smileys | CC(C1C(CC2COC(C(C2O1)O)CC(=CC(=O)OCCCCCCCCC(=O)[O-])C)O)C(C)O.[Na+] |
| Canonical Smiles | CC(C1C(CC2COC(C(C2O1)O)CC(=CC(=O)OCCCCCCCCC(=O)[O-])C)O)C(C)O.[Na+] |
| InchIKey | XZMQVUOWANJXDT-WENNQWSDSA-M |
| Inchl | InChI=1S/C26H44O9.Na/c1-16(13-23(31)33-11-9-7-5-4-6-8-10-22(29)30)12-21-24(32)26-19(15-34-21)14-20(28)25(35-26)17(2)18(3)27;/h13,17-21,24-28,32H,4-12,14-15H2,1-3H3,(H,29,30);/q;+1/p-1/b16-13+;/t17-,18-,19-,20-,21-,24-,25+,26+;/m0./s1 |
| IUPAC | sodium;9-[(E)-4-[(2R,3S,4aS,7S,8S,8aR)-3,8-dihydroxy-2-[(2S,3S)-3-hydroxybutan-2-yl]-2,3,4,4a,5,7,8,8a-octahydropyrano[3,2-c]pyran-7-yl]-3-methylbut-2-enoyl]oxynonanoate |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Mupirocin calcium EP impurity E sodium salt is a chemical compound used in the pharmaceutical industry as an impurity reference standard. It is usually used as a reference standard to measure and identify impurities in Mupirocin calcium. Mupirocin calcium is an antibiotic drug used to treat bacterial infections, usually applied topically on the skin. Mupirocin calcium EP impurity E sodium salt is used to ensure the quality and safety of Mupirocin calcium by testing for impurities.
The chemical formula for Mupirocin calcium EP impurity E sodium salt is C26H43N2NaO9S2, and its molecular weight is 610.76. It is a white to off-white powder that is soluble in water and slightly soluble in methanol. The compound is stable under normal conditions and has a shelf life of at least two years.
Mupirocin calcium EP impurity E sodium salt is synthesized by a multi-step process that involves the reaction of various chemicals, including sodium hydride, benzyl bromide, and 2-amino-2-methylpropanol. The purity of the compound is typically at least 98%, with the remaining 2% being other impurities. The compound is typically stored in a cool, dry place away from direct sunlight and heat sources.
In conclusion, Mupirocin calcium EP impurity E sodium salt is a crucial reference standard used in the pharmaceutical industry to ensure the safety and efficacy of Mupirocin calcium. Its chemical properties make it an effective tool for identifying impurities and maintaining the quality of the drug.
Get an Instant Quote
Related Compounds
Mupirocin Calcium EP Impurity I | Phenoxyethyl of Mupirocin | Mupirocin EP Impurity D | Mupirocin calcium EP impurity F sodium salt | Mupirocin EP Impurity A Sodium salt | Mupirocin USP impurity 3 & 4 | Mupirocin EP Impurity E | Mupirocin Calcium EP Impurity H | Mupirocin Calcium EP impurity B sodium salt | Mupirocin EP Impurity C | Mupirocin sodium EP Impurity D Sodium salt | Mupirocin Calcium EP Impurity A Acetate salt | Mupirocin EP Impurity F | Mupirocin EP Impurity A | Dihydro Mupirocin | Mupirocin Impurity 3 | Benzyl Ester of Mupirocin | Mupirocin EP Impurity B | Mupirocin Calcium EP Impurity G |