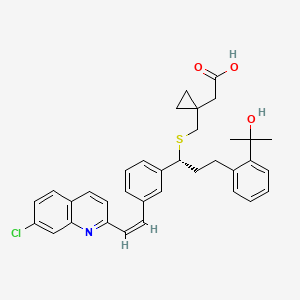
Montelukast EP impurity G
| Product Name | Montelukast EP impurity G |
|---|---|
| Alternate Names | Montelukast Impurities, Impurities of Montelukast |
| CAT No. | CS-O-11861 |
| CAS No. | 774538-96-4 |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | 586.18 g/mol |
| Mol. For. | C₃₅H₃₆ClNO₃S |
| Hazardous | This is a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Montelukast |
| Purity | 95% |
| Therapeutic | Anti-Asthma / COPD |
| Smileys | OC(CC1(CC1)CS[C@@H](C2=CC(/C=C\C3=NC(C=C(Cl)C=C4)=C4C=C3)=CC=C2)CCC(C=CC=C5)=C5C(C)(O)C)=O |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Montelukast EP impurity G is a chemical compound that is used in the pharmaceutical industry as a reference standard for the analysis of montelukast in various drug formulations. Montelukast is a selective leukotriene receptor antagonist that is used in the treatment of asthma and seasonal allergies. Montelukast EP impurity G is an important reference standard as it helps in the identification and quantification of montelukast in different drug formulations.
The chemical name of Montelukast EP impurity G is (R)-2-[[[1-[3-[2-(7-chloro-2-quinolinyl)ethenyl]phenyl]-3-[2-(1-hydroxy-1-methylethyl)phenyl]propyl]thio]methyl]butyric acid. Its molecular formula is C30H32ClNO3S and its molecular weight is 525.11 g/mol.
Montelukast EP impurity G is a solid material that is soluble in organic solvents like methanol, ethanol, and acetonitrile. It is stable under normal conditions and is used as a reference standard in analytical methods like high-performance liquid chromatography (HPLC) and gas chromatography-mass spectrometry (GC-MS).
In conclusion, Montelukast EP impurity G is an important reference standard used in the pharmaceutical industry for the analysis of montelukast in different drug formulations. It helps in the identification and quantification of montelukast and is used in various analytical methods.
Get an Instant Quote
Related Compounds
Des[3-[[(1-Carboxymethyl)cyclopropyl]methyl]thio]-2-propenyl Montelukast Mesylate | Montelukast cyclopropaneacetamide (Impurity) | Montelukast Dehydro Impurity | Cyclopropane-1,1- diylbis(methylene) dimethanesulfonate | Montelukast EP Impurity C (R isomer) | Montelukast Cyclizate Ether impurity | Montelukast Dicarboxylic Acid | Montelukast Gem-dimethylmethylene Analogue | Montelukast (3R)-Hydroxy Propanol | Montelukast EP impurity I | Montelukast EP impurity F | Montelukast Methyl Ester | Montelukast EP Impurity C (Mixture of isomers) | Montelukast Sulphoxide | Montelukast EP Impurity B | Montelukast Chloroalcohol impurity | Montelukast Diol | Montelukast Sulfone | Montelukast methanesulfonate IMPURITY | Montelukast EP Impurity D+E (Michael Adduct 1+2) | Montelukast Bis-sulfide | Montelukast EP impurity H | Montelukast EP impurity D | Montelukast Cyclopropaneacetonitrile | Montelukast EP impurity A | Montelukast sulfone N-Oxide | Montelukast Acid impurity | ent-Montelukast Sodium Salt | Montelukast 3-Oxo Propanol Impurity | Montelukast sodium racemate | Dihydro Montelukast Sodium Salt | Montelukast Ketocarbinol Impurity | Montelukast EP impurity E | Dihydro Montelukast Sodium Salt |