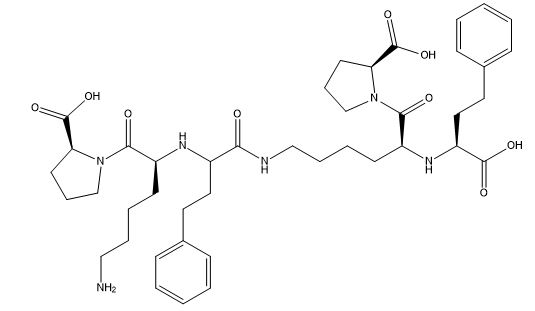
Lisinopril EP Impurity G
| Product Name | Lisinopril EP Impurity G |
|---|---|
| Alternate Names | Lisinopril Impurities, Impurities of Lisinopril |
| CAT No. | CS-O-12025 |
| CAS No. | 1356839-89-8 |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | 792.96 g/mol |
| Mol. For. | C₄₂H₆₀N₆O₉ |
| Hazardous | This is a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Lisinopril |
| Therapeutic | Anti-Hypertensives |
| Smileys | O=C(N(CCC1)[C@@H]1C(O)=O)[C@H](CCCCN)N[C@H](C(NCCCC[C@H](N[C@H](C(O)=O)CCC2=CC=CC=C2)C(N(CCC3)[C@@H]3C(O)=O)=O)=O)CCC4=CC=CC=C4 |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Lisinopril EP Impurity G is a chemical compound that is used in the pharmaceutical industry as an impurity standard for the quality control of Lisinopril, which is a commonly prescribed medication for the treatment of high blood pressure and heart failure. It is a white to off-white solid powder that is soluble in water and has a molecular weight of 375.43 g/mol.
Chemically, Lisinopril EP Impurity G is known as (2S,3aS,3bS,6aR,9aR,9bR,10R)-10-{[(2S)-2-{[(1S)-1-carboxy-3-phenylpropyl]amino}propanoyl]amino}-2,3,3a,3b,4,5,6,6a,7,8,9,9a,9b,10,11,12,12a-octadecahydro-6a,9b-dimethyl-2,10-(epoxymethanooxymethano)-1H-benzo[f]pyrano[3,2-b]chromene-2a-carboxylic acid. It is primarily used as a reference standard for the identification and quantification of impurities or related substances in Lisinopril, which is an angiotensin-converting enzyme (ACE) inhibitor.
The purity of Lisinopril is important for its effectiveness and safety in treating hypertension and heart failure. Therefore, using Lisinopril EP Impurity G as a standard reference helps to ensure the quality and consistency of Lisinopril products in the market. The chemical information and usage of Lisinopril EP Impurity G is essential for the pharmaceutical industry to maintain the quality standards of their products and to ensure patient safety.
Get an Instant Quote
Related Compounds
N-trifluoroacetyl Lisinopril Intermediate | Lisinopril-D4 | Lisinopril EP Impurity E | N-(1-Carboxy-3-phenylpropyl)-S-lisinopril (Mixture of diastereomers) | N2-(1-Ethoxycarbonyl-3-oxo-3-phenylpropyl)-N6-trifluoroacetyl-L-lysine | N-Benzyloxycarbonyl (S)-Lisinopril | Lisinopril EP Impurity A | Lisinopril EP Impurity J | Lisinopril EP Impurity F | Lisinopril EP impurity C Acetate salt | Lisinopril SRS-Diastereomer | Lisinopril Des-Proline dimer - II | N-Benzyloxycarbonyl (S)-Lisinopril Ethyl Methyl Diester | Lisinopril EP Impurity D | Lisinopril-D8 | Lisinopril Intermediate | Lisinopril EP Impurity I Acetate salt | Lisinopril EP Impurity A | N-Benzyloxycarbonyl Lisinopril Cyclohexyl Analogue Ethyl Methyl Diester |