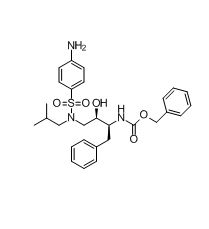
Darunavir СВZ amino impurity
| Product Name | Darunavir СВZ amino impurity |
|---|---|
| Alternate Names | Darunavir Impurities, Impurities of Darunavir |
| CAT No. | CS-O-12038 |
| CAS No. | 159005-61-5 |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | 525.66 g/mol |
| Mol. For. | C₂₈H₃₅N₃O₅S |
| Hazardous | This is a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Darunavir |
| Purity | 95% |
| Therapeutic | Antiretroviral / Anti-HIV |
| Smileys | O=C(N[C@H]([C@@H](CN(S(=O)(C1=CC=C(C=C1)N)=O)CC(C)C)O)CC2=CC=CC=C2)OCC3=CC=CC=C3 |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Darunavir is a drug used to treat human immunodeficiency virus (HIV) infections. It works by inhibiting the activity of the viral protease enzyme, which is essential for the virus to replicate and mature. Darunavir is available in various formulations, including tablets, oral suspension, and extended-release tablets. However, during the manufacturing process, impurities may be introduced into the drug substance. One such impurity is the Darunavir СВZ amino impurity.
The Darunavir СВZ amino impurity is a chemical byproduct that occurs during the synthesis of Darunavir. It is a small molecule that contains an amino group and a carboxyl group. This impurity is considered to be a potential genotoxic and mutagenic agent, and therefore, its presence in Darunavir needs to be controlled.
The maximum allowable level of the Darunavir СВZ amino impurity in Darunavir drug substance is 0.15%. The impurity can be removed by various purification techniques, including chromatography, crystallization, and recrystallization.
In conclusion, the Darunavir СВZ amino impurity is a chemical byproduct that can be present in Darunavir drug substance. Its presence needs to be controlled due to its potential genotoxic and mutagenic properties. Various purification techniques can be used to remove this impurity and ensure the safety and efficacy of Darunavir.
Get an Instant Quote
Related Compounds
Darunavir Impurity 16 | Darunavir Isomer 2 | Darunavir СВZ furan impurity | Darunavir Isomer 1 | Darunavir Urea impurity | Darunavir Impurity 11 | Darunavir Amine dimer impurity | Darunavir Impurity 38 | N-[3S-benzyloxycarbonylamino-2R-hydroxy-4-phenyl]-N-isobutylamine | Darunavir Nitro complex impurity | Darunavir impurity B | Darunavir Impurity 7 (S,S-Isomer) | Darunavir Difuranyl impurity | Darunavir Impurity 23 | Darunavir furan dimer impurity | Darunavir Carbamic Acid Methyl Ester | Darunavir Impurity A Enantiomer | Darunavir Impurity 46 | Darunavir Impurity 8 (R,R-Isomer) | Darunavir N-methyl urea impurity | Darunavir Impurity 10 | Darunavir СВZ urea impurity | Darunavir Diamino impurity | Darunavir Impurity D |