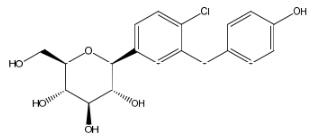
Empagliflozin Destetrahydrofuran Impurity
| Product Name | Empagliflozin Destetrahydrofuran Impurity |
|---|---|
| Alternate Names | Empagliflozin Impurities, Impurities of Empagliflozin |
| CAT No. | CS-O-12074 |
| CAS No. | 864070-37-1 |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | 380.82 g/mol |
| Mol. For. | C₁₉H₂₁ClO₆ |
| Hazardous | This is a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Empagliflozin |
| Purity | Not less than 95 % |
| Therapeutic | Anti-Diabetic |
| Smileys | O[C@H]1[C@H](C2=CC(CC3=CC=C(O)C=C3)=C(Cl)C=C2)O[C@H](CO)[C@@H](O)[C@@H]1O |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Empagliflozin Destetrahydrofuran Impurity is a chemical compound that is often used in the pharmaceutical industry as an analytical standard. It is a degradation product of Empagliflozin, which is a medication that is used to treat type 2 diabetes. This impurity is formed when Empagliflozin is exposed to high temperatures or other conditions that cause it to break down.
Empagliflozin Destetrahydrofuran Impurity is a white to off-white powder that is soluble in water and other polar solvents. Its molecular formula is C23H27ClO7, and its molecular weight is 452.91 g/mol. The compound has a melting point of around 155-160 °C and a boiling point of around 566.6 °C at 760 mmHg.
While Empagliflozin Destetrahydrofuran Impurity is not intended for human consumption, it is an important tool for quality control in the manufacturing of Empagliflozin. By using this impurity as an analytical standard, manufacturers can ensure that their Empagliflozin products meet the required purity standards and are safe for human use.
In conclusion, Empagliflozin Destetrahydrofuran Impurity is a vital component of the pharmaceutical industry that serves an important role in quality control. Its chemical properties and usage allow for the safe and effective production of medications like Empagliflozin, which help to manage and treat conditions like type 2 diabetes.
Get an Instant Quote
Related Compounds
Empagliflozin Iodo R-furan impurity | Empagliflozin Impurity 26 | Empagliflozin S-Furanose | Empagliflozin impurity D | Empagliflozin Des bromomethoxy | Empagliflozin Iodo impurity | Empagliflozin impurity A | Empagliflozin Dimer Impurity | Empagliflozin Silyl Impurity | Hydroxy empagliflozin | Empagliflozin impurity B | Empagliflozin MonoAcetyl Impurity | Empagliflozin Impurity 50 | Empagliflozin R-Furanose | Empagliflozin Recemic mixture | Empagliflozin Impurity 46 | Empagliflozin Enantiomer Impurity | Empagliflozin Bromo Impurity | Empagliflozin Open Ring Impurity | Empagliflozin Nosyl ester hydroxy furan impurity | Empagliflozin Sulfonate Impurity | Empagliflozin Impurity 40 | Empagliflozin M468 | Empagliflozin Methyl Acetate | Empagliflozin Enantiomer Impurity | Methoxy Empagliflozin R-Furanose | Empagliflozin Impurity 5 | Empagliflozin Acetyl impurity | Empagliflozin Impurity POE | Empagliflozin Impurity 82 | Empagliflozin Methoxy impurity | O-Destetrahydrofuran O-Pentaacetyl Empagliflozin | Empagliflozin alpha Isomer | Empagliflozin Intermediate-A | Empagliflozin Impurity E | Empagliflozin Dichloro impurity | Empagliflozin Nosyl impurity | Empagliflozin Impurity 52 | DIMETHOXY EMPAGLIFLOZIN | Empagliflozin impurity C | Empagliflozin M464 | Empagliflozin Methoxy Intermediate | Empagliflozin Impurity 62 | Empagliflozin Diol impurity (S-Isomer) | Empagliflozin Sugar Dimer | Empagliflozin Impurity 2 | Empagliflozin Impurity 4 | Empagliflozin L- Glucono Diastereomer | Empagliflozin Impurity II |