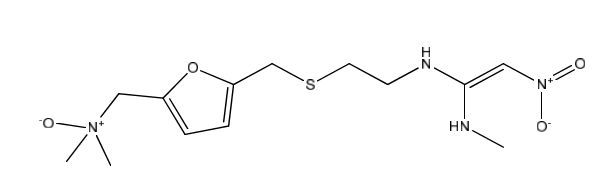
Ranitidine EP Impurity E
| Product Name | Ranitidine EP Impurity E |
|---|---|
| Alternate Names | Ranitidine Impurities, Impurities of Ranitidine |
| CAT No. | CS-O-13640 |
| CAS No. | 73857-20-2 |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | 330.40 g/mol |
| Mol. For. | C₁₃H₂₂N₄O₄S |
| Hazardous | This is a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Ranitidine |
| Purity | 95% |
| Therapeutic | Anti ulcer |
| Smileys | C[N](CC1=CC=C(CSCCN/C(NC)=C/[N](=O)=O)O1)(C)=O |
| Canonical Smiles | CNC(=C[N+](=O)[O-])NCCSCC1=CC=C(O1)C[N+](C)(C)[O-] |
| InchIKey | DFJVUWAHTQPQCV-UHFFFAOYSA-N |
| Inchl | InChI=1S/C13H22N4O4S/c1-14-13(8-16(18)19)15-6-7-22-10-12-5-4-11(21-12)9-17(2,3)20/h4-5,8,14-15H,6-7,9-10H2,1-3H3 |
| IUPAC | N,N-dimethyl-1-[5-[2-[[1-(methylamino)-2-nitroethenyl]amino]ethylsulfanylmethyl]furan-2-yl]methanamine oxide |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Ranitidine EP Impurity E, also known as N-nitrosodimethylamine (NDMA), is a chemical compound that has been identified as a potential carcinogen. It is a yellow oily liquid that is soluble in water and organic solvents. NDMA is classified as a Group 2A carcinogen by the International Agency for Research on Cancer (IARC), which means that it is probably carcinogenic to humans.
NDMA has been found as a contaminant in many pharmaceutical products, including ranitidine, which is a medication that is used to treat heartburn and acid reflux. Ranitidine EP Impurity E is formed during the manufacturing process of ranitidine and can be present in trace amounts in the final product.
The use of NDMA in pharmaceuticals has been strictly regulated by health authorities around the world due to its carcinogenic properties. The United States Food and Drug Administration (FDA) has issued multiple recalls of ranitidine-containing products due to the presence of NDMA.
Due to the potential health risks associated with NDMA, it is important for pharmaceutical companies to monitor and control the levels of this impurity in their products. Ranitidine EP Impurity E should be avoided and kept at low levels to ensure the safety and efficacy of pharmaceutical products containing ranitidine.
Get an Instant Quote
Related Compounds
Ranitidine EP Impurity J | Ranitidine EP Impurity F | Desmethyl Ranitidine | Ranitidine N-Nitroso Impurity | Ranitidine EP Impurity D | Ranitidine Impurity F.HCl | Ranitidine Related Compound A | Ranitidine EP Impurity H | Ranitidine EP Impurity K | Ranitidine EP Impurity I | Ranitidine oxalic acid | Ranitidine EP Impurity G | Ranitidine Impurity 2 | Ranitidine EP impurity A | Ranitidine EP Impurity B | Ranitidine N,S-Dioxide |