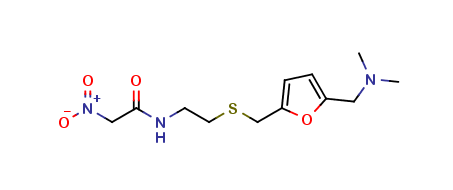
Ranitidine EP Impurity D
| Product Name | Ranitidine EP Impurity D |
|---|---|
| Alternate Names | Ranitidine Impurities, Impurities of Ranitidine |
| CAT No. | CS-O-13642 |
| CAS No. | 117846-02-3 |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | 301.36 g/mol |
| Mol. For. | C₁₂H₁₉N₃O₄S |
| Hazardous | This is a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Ranitidine |
| Purity | Not less than 95 % |
| Therapeutic | Anti ulcer |
| Smileys | CN(C)CC1=CC=C(O1)CSCCNC(=O)C[N+](=O)[O-] |
| Canonical Smiles | CN(C)CC1=CC=C(O1)CSCCNC(=O)C[N+](=O)[O-] |
| InchIKey | PFYRWZJFERNHFP-UHFFFAOYSA-N |
| Inchl | InChI=1S/C12H19N3O4S/c1-14(2)7-10-3-4-11(19-10)9-20-6-5-13-12(16)8-15(17)18/h3-4H,5-9H2,1-2H3,(H,13,16) |
| IUPAC | N-[2-[[5-[(dimethylamino)methyl]furan-2-yl]methylsulfanyl]ethyl]-2-nitroacetamide |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Ranitidine EP Impurity D is a chemical compound that is commonly used in the pharmaceutical industry for the manufacturing of Ranitidine, a medication used to treat stomach ulcers and acid reflux. Ranitidine EP Impurity D is a by-product or impurity that is formed during the synthesis of Ranitidine. It is a white to off-white powder that is soluble in water and organic solvents.
The chemical name of Ranitidine EP Impurity D is N-nitroso-N-methyl-4-amino-5-nitroso-2-methoxybenzamide. It is a derivative of benzamide and contains a nitroso group, which is responsible for its reactivity and potential toxicity. Studies have shown that N-nitroso compounds can cause DNA damage and increase the risk of cancer.
Due to its potential toxicity, Ranitidine EP Impurity D is strictly regulated by regulatory authorities such as the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA). The maximum allowable limit of Ranitidine EP Impurity D in Ranitidine products is set at 0.1% by weight.
In conclusion, Ranitidine EP Impurity D is an important chemical compound in the pharmaceutical industry, but its potential toxicity highlights the importance of strict regulation and quality control in manufacturing processes.
Get an Instant Quote
Related Compounds
Ranitidine EP Impurity H | Ranitidine EP Impurity K | Ranitidine Impurity 2 | Ranitidine Related Compound A | Ranitidine EP impurity A | Ranitidine EP Impurity G | Ranitidine EP Impurity F | Ranitidine EP Impurity E | Ranitidine Impurity F.HCl | Ranitidine N,S-Dioxide | Ranitidine EP Impurity J | Ranitidine EP Impurity B | Ranitidine oxalic acid | Ranitidine N-Nitroso Impurity | Ranitidine EP Impurity I | Desmethyl Ranitidine |