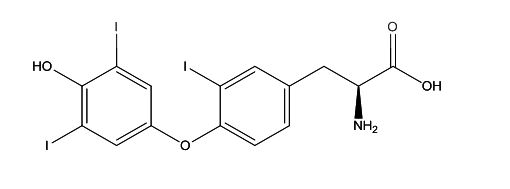
Levothyroxine EP Impurity K
| Product Name | Levothyroxine EP Impurity K |
|---|---|
| Alternate Names | Levothyroxine Impurities, Impurities of Levothyroxine |
| CAT No. | CS-O-14067 |
| CAS No. | 5817-39-0 |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | 650.97 g/mol |
| Mol. For. | C₁₅H₁₂I₃NO₄ |
| Hazardous | This is a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Levothyroxine |
| Purity | 95% |
| Smileys | IC1=C(C=CC(C[C@H](N)C(O)=O)=C1)OC2=CC(I)=C(O)C(I)=C2 |
| Canonical Smiles | C1=CC(=C(C=C1CC(C(=O)O)N)I)OC2=CC(=C(C(=C2)I)O)I |
| InchIKey | HZCBWYNLGPIQRK-LBPRGKRZSA-N |
| Inchl | InChI=1S/C15H12I3NO4/c16-9-3-7(4-12(19)15(21)22)1-2-13(9)23-8-5-10(17)14(20)11(18)6-8/h1-3,5-6,12,20H,4,19H2,(H,21,22)/t12-/m0/s1 |
| IUPAC | (2S)-2-amino-3-[4-(4-hydroxy-3,5-diiodophenoxy)-3-iodophenyl]propanoic acid |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Levothyroxine EP Impurity K is a chemical compound that is commonly used in the pharmaceutical industry as a reference standard for the testing and analysis of Levothyroxine. Levothyroxine is a synthetic thyroid hormone that is used to treat hypothyroidism, a condition where the thyroid gland produces insufficient hormones. The purity of Levothyroxine is crucial for its effective therapeutic use, and the presence of impurities can affect its potency and efficacy.
Levothyroxine EP Impurity K is a known impurity that is often present in Levothyroxine formulations. It is a derivative of 3,5-diiodothyronine, which is a natural hormone produced by the thyroid gland. The chemical formula of Levothyroxine EP Impurity K is C15H11I2NO4, and its molecular weight is 528.06 g/mol.
In terms of usage, Levothyroxine EP Impurity K is primarily used as a reference standard for the analysis of Levothyroxine formulations. It is used by pharmaceutical companies and research laboratories to determine the purity and quality of Levothyroxine products. The compound is also used to develop analytical methods for the detection and quantification of Levothyroxine and its impurities.
Overall, Levothyroxine EP Impurity K plays an important role in the pharmaceutical industry by ensuring the quality and efficacy of Levothyroxine products. Its chemical properties and usage make it a valuable tool for the development and testing of Levothyroxine formulations.
Get an Instant Quote
Related Compounds
levothyroxine Dimer | Levothyroxine-4-Lactose Maillard Impurity | Levothyroxine Ethyl Ester | Levothyroxine EP Impurity C | Levothyroxine- Impurity NMP | Levothyroxine - Impurity G | Levothyroxine EP Impurity I | Levothyroxine- Impurity 5 | Levothyroxine Monochloro Impurity | Levothyroxine N- formamide Impurity | Levothyroxine EP Impurity B | O-(4-Hydroxy-3-iodophenyl) Levothyroxine | Levothyroxine Lactose Adduct | Levothyroxine Impurity 30 Sodium Salt | Levothyroxine Lactose Adduct (N-lactoside) | Levothyroxine Sodium EP Impurity J | Levothyroxine Glucose Adduct | TRIIODO-L-THYRONINE AMPOLA 100 µG/ML | Levothyroxine EP Impurity E | Levothyroxine Lactose Adduct (O-lactoside) | Levothyroxine EP Impurity H | Levothyroxine disodium salt | Levothyroxine Related Compound 10 |