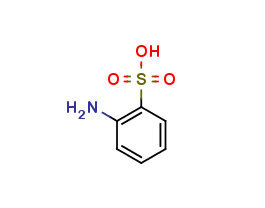
Mesalazine EP Impurity O
| Product Name | Mesalazine EP Impurity O |
|---|---|
| Alternate Names | Mesalazine Impurities, Impurities of Mesalazine |
| CAT No. | CS-O-14084 |
| CAS No. | 88-21-1 |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | 173.19 g/mol |
| Mol. For. | C₆H₇NO₃S |
| Hazardous | This is a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Mesalazine |
| Purity | 95% |
| Smileys | O=[S](O)(C(C=CC=C1)=C1N)=O |
| Canonical Smiles | C1=CC=C(C(=C1)N)S(=O)(=O)O |
| InchIKey | ZMCHBSMFKQYNKA-UHFFFAOYSA-N |
| Inchl | InChI=1S/C6H7NO3S/c7-5-3-1-2-4-6(5)11(8,9)10/h1-4H,7H2,(H,8,9,10) |
| IUPAC | 2-aminobenzenesulfonic acid |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Mesalazine EP Impurity O is a chemical compound that is commonly used in the pharmaceutical industry as a reference standard or impurity for the analysis of mesalazine or 5-aminosalicylic acid (5-ASA). Mesalazine EP Impurity O is also known as 2-Amino-4-hydroxybenzoic acid or Gentisic acid, and its chemical formula is C7H7NO3.
This compound is a derivative of salicylic acid and is a white to light yellow crystalline powder that is soluble in water and ethanol. The melting point of Mesalazine EP Impurity O is around 210-215°C. This impurity is commonly used in the development and quality control of mesalazine-containing products and is also used as a reference standard for the analysis of other related compounds.
Mesalazine EP Impurity O is an important tool in the quality control of mesalazine-containing formulations, as it helps to ensure that the product is free from impurities that could affect its efficacy or safety. The use of this compound in the pharmaceutical industry is regulated by various agencies such as the United States Pharmacopeia (USP) and the European Pharmacopoeia (EP).
In conclusion, Mesalazine EP Impurity O is an important reference standard in the pharmaceutical industry, which is used for the development and quality control of mesalazine-containing products. Its chemical properties and usage make it an essential tool in the manufacturing and analysis of these formulations.
Get an Instant Quote
Related Compounds
Mesalazine Impurity 14 | Mesalazine EP Impurity M | Mesalazine EP Impurity I | Mesalazine Impurity R | Mesalazine EP Impurity C | Mesalazine EP Impurity G | Mesalazine impurity A | Mesalazine Impurity L | Mesalazine EP Impurity J DiHCl |