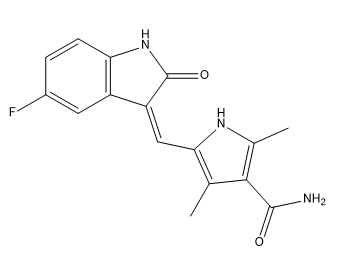
Sunitinib Impurity I
| Product Name | Sunitinib Impurity I |
|---|---|
| Alternate Names | Sunitinib Impurities, Impurities of Sunitinib |
| CAT No. | CS-O-14530 |
| CAS No. | 1186651-51-3 |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | 299.30 g/mol |
| Mol. For. | C₁₆H₁₄FN₃O₂ |
| Hazardous | This is a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Sunitinib |
| Therapeutic | Anti-Cancer / Oncology |
| Smileys | O=C1/C(C2=CC(F)=CC=C2N1)=C\C(NC(C)=C3C(N)=O)=C3C |
| Canonical Smiles | CC1=C(NC(=C1C(=O)N)C)C=C2C3=C(C=CC(=C3)F)NC2=O |
| InchIKey | WAHDFFHPBSCPOQ-WDZFZDKYSA-N |
| Inchl | InChI=1S/C16H14FN3O2/c1-7-13(19-8(2)14(7)15(18)21)6-11-10-5-9(17)3-4-12(10)20-16(11)22/h3-6,19H,1-2H3,(H2,18,21)(H,20,22)/b11-6- |
| IUPAC | 5-[(Z)-(5-fluoro-2-oxo-1H-indol-3-ylidene)methyl]-2,4-dimethyl-1H-pyrrole-3-carboxamide |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Sunitinib Impurity I is a chemical compound that is used in the pharmaceutical industry as a reference standard for the analysis of sunitinib malate, a tyrosine kinase inhibitor drug used to treat cancer. The impurity is a byproduct of the synthesis of sunitinib malate and can be present in trace amounts in the drug product.
Sunitinib Impurity I has a chemical formula of C21H16ClN3O2 and a molecular weight of 375.83 g/mol. It is a yellow solid with a melting point range of 126-129°C. The compound is soluble in organic solvents such as DMSO, methanol, and acetonitrile.
Chemically, Sunitinib Impurity I is a derivative of the parent compound sunitinib, with a chlorine atom substituted for a methyl group. The compound has been characterized using various analytical techniques such as nuclear magnetic resonance spectroscopy, mass spectrometry, and infrared spectroscopy.
In the pharmaceutical industry, Sunitinib Impurity I is used as a reference standard for the analysis of sunitinib malate. It is used to verify the purity of the drug substance and to ensure that the drug product meets regulatory requirements for quality and safety. The impurity can also be used in the development of analytical methods for the analysis of sunitinib malate.
Get an Instant Quote
Related Compounds
Sunitinib Ketone Impurity | Sunitinib N-3-(Dimethylamino)propyl impurity | N-Desethyl Sunitinib | Sunitinib Impurity C | Sunitinib Impurity 10 | Sunitinib Impurity 8 | N-Desethylsunitinib-d4 TFA Salt | N-Desethyl N-Nitroso Sunitinib impurity | Sunitinib Impurity 1 | Sunitinib Carboxylic Acid | Sunitinib Impurity 18 | N-Boc-N,N-didesethyl Sunitinib | Sunitinib Impurity II (SNT 700) | sunitinib Impurity B | Sunitinib Impurity 7 | Sunitinib formyl impurity | N-Desethylsunitinib TFA Salt | Sunitinib Impurity H |