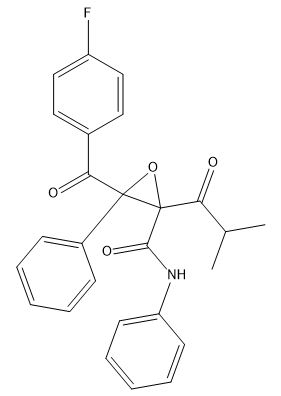
Atorvastatin Impurity D
| Chemical Name | Atorvastatin Impurity D |
|---|---|
| Alternate Names | Atorvastatin Impurities, Impurities of Atorvastatin |
| CAT No. | CS-O-14632 |
| CAS Registry# | 148146-51-4 |
| Category | Impurities |
| Stock | Enquire |
| Mol. Wt. | 431.46 g/mol |
| Mol. For. | C₂₆H₂₂FNO₄ |
| Hazardous | This is a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Atorvastatin |
| Purity | Not less than 90 % |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Atorvastatin Impurity D is a chemical compound that is used in the pharmaceutical industry for the analysis and testing of Atorvastatin Calcium. Atorvastatin Calcium is a medication that is used to lower cholesterol levels in the blood, and the presence of impurities in the drug can affect its effectiveness and safety. Atorvastatin Impurity D is one of the impurities that can be present in Atorvastatin Calcium, and its detection and quantification are crucial for ensuring the quality and purity of the drug.
Atorvastatin Impurity D is a white to off-white powder that has a molecular weight of 490.67 g/mol. Its chemical formula is C28H42O3, and it has a melting point of 144-146°C. Atorvastatin Impurity D is a derivative of Atorvastatin, and it is formed during the synthesis process of the drug. The impurity is typically present in small quantities, and its level of concentration is usually measured in parts per million (ppm).
The detection and quantification of Atorvastatin Impurity D can be performed using various analytical techniques such as high-performance liquid chromatography (HPLC), gas chromatography (GC), and mass spectrometry (MS). These methods are highly sensitive and accurate, and they can detect and quantify impurities at very low concentrations.
In conclusion, Atorvastatin Impurity D is a chemical compound that is used in the pharmaceutical industry for the analysis and testing of Atorvastatin Calcium. Its detection and quantification are crucial for ensuring the quality and purity of the drug, and various analytical techniques can be used for this purpose.
Get an Instant Quote
Related Compounds
Atorvastatin Impurity 17 | Difluoro Atorvastatin | Atorvastatin EP Impurity A (Sodium Salt) | Atorvastatin Amide | Allyl Ester of Atorvastatin Cyclic (Fluorophenyl) Impurity | Atorvastatin Impurity F | Atorvastatin EP Impurity A (Sodium salt) | Atorvastatin 5-O-Methyl Sodium | Atorvastatin Dehydro Lactone | Atorvastatin Acid Sodium salt | Atorvastatin EP Impurity G Sodium Salt | Atorvastatin Diepoxide Impurity | Atorvastatin 3-Deoxyhept-2Z-Enoic Acid Sodium Salt | Atorvastatin Acetonide | Atorvastatin EP Impurity C Sodium salt | Atorvastatin Triamino impurity | Atorvastatin Epoxy Pyrrolooxazin tricyclic Calcium Salt | Atorvastatin Ethyl Ester | Atorvastatin EP Impurity C | Atorvastatin Lactam Sodium Salt Impurity | Atorvastatin Epoxy Tetrahydrofuran Analog | Atorvastatin Related compound B | Atorvastatin Pyrrolidone Impurity | Atorvastatin Impurity 21 | Atorvastatin Acid Methyl Ester | Atorvastatin EP Impurity B Calcium salt | Atorvastatin EP Impurity A | Atorvastatin Impurity 1 | Atorvastatin EP Impurity I | Atorvastatin Calcium Propylene Glycol Solvate | Atorvastatin Impurity 25 | Atorvastatin Pyrrolidone Phenanthrene Calcium | Atorvastatin EP Impurity B | sodium (3R,5R)-7-(2-(4-fluorophenyl)-5-isopropyl-3-phenyl-1H-pyrrol-1-yl)-3,5-dihydroxyheptanoate | Atorvastatin Impurity 19 | Atorvastatin 2-Fluoro t-Butyl Ester | Atorvastatin Tri-acetonide tert-Butyl Ester | Atorvastatin EP Impurity J (Calcium salt) | Atorvastatin Oxirane Impurity | N-Nitroso Atorvastatin Impurity | Atorvastatin diacid impurity | Atorvastatin Lactam Calcium Salt Impurity | Atorvastatin Lactam Phenanthrene | Atorvastatin EP Impurity F (Ca Salt) | Atorvastatin Diamino impurity | Atorvastatin Cyclic Fluorophenyl calcium Salt Impurity | Atorvastatin EP Impurity D | Atorvastatin 2-Hydroxy Sodium | Atorvastatin Di-acetonide tert-Butyl Ester | Atorvastatin Cyclic (Fluorophenyl) Impurity | Atorvastatin Lactone 3-O-Methyl Ether | Atorvastatin Isopropyl Ester | Atorvastatin Dehydro Sodium Salt (E/Z mixture) | Allyl Ester of Atorvastatin Cyclic (Isopropyl) Impurity | Atorvastatin Lactam Impurity calcium salt | Atorvastatin Acid t-Butyl Ester | Atorvastatin 3-Deoxyhept-2E-Enoic Acid | Atorvastatin EP Impurity P (Sodium salt) | Atorvastatin EP Impurity E Sodium salt | Atorvastatin Lactone Diepoxide | Atorvastatin Pyrrolidone Phenanthrene Sodium | Atorvastatin EP Impurity G (3-O-methyl Atorvastatin calcium salt) | Atorvastatin Cyclic (Fluorophenyl) Impurity | Atorvastatin 2-Fluoro Analog | Atorvastatin Diketoene Impurity | Atorvastatin 3-Fluoro Analog | Atorvastatin EP Impurity P calcium Salt | Atorvastatin EP Impurity Q sodium salt | Atorvastatin Cyclic (Fluorophenyl) Sodium | Atorvastatin Diepoxide Calcium Salt |