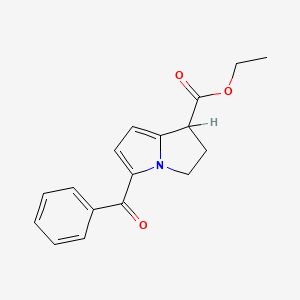
Ketorolac EP Impurity J
| Product Name | Ketorolac EP Impurity J |
|---|---|
| Alternate Names | Ketorolac Impurities, Impurities of Ketorolac |
| CAT No. | CS-O-14764 |
| CAS No. | 108061-03-6 |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | 283.32 g/mol |
| Mol. For. | C17H17O3N |
| Hazardous | This is a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Ketorolac |
| Therapeutic | Anti-Migraines |
| Smileys | O=C(C1=CC=CC=C1)C2=CC=C3N2CCC3C(OCC)=O |
| Canonical Smiles | CCOC(=O)C1CCN2C1=CC=C2C(=O)C3=CC=CC=C3 |
| InchIKey | ZXHMVDVCMQIUCB-UHFFFAOYSA-N |
| Inchl | InChI=1S/C17H17NO3/c1-2-21-17(20)13-10-11-18-14(13)8-9-15(18)16(19)12-6-4-3-5-7-12/h3-9,13H,2,10-11H2,1H3 |
| IUPAC | ethyl 5-benzoyl-2,3-dihydro-1H-pyrrolizine-1-carboxylate |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Ketorolac is a non-steroidal anti-inflammatory drug (NSAID) used to treat moderate to severe pain. Ketorolac EP Impurity J, also known as (5Z,13E,15S)-15-hydroxy-5,13-docosadienoic acid, is an impurity found in the synthesis of ketorolac.
Ketorolac EP Impurity J is not used directly in medical treatments, but its chemical information is important for quality control purposes. It is essential to ensure that the amount of the impurity is below a certain level to ensure the safety and efficacy of the ketorolac drug.
The structure of Ketorolac EP Impurity J is a long chain fatty acid with a hydroxyl group and a double bond. It is an unsaturated fatty acid, which means it has one or more double bonds in its structure. The double bonds make the molecule more reactive and prone to oxidation, which can lead to the formation of other impurities.
Analytical techniques such as high-performance liquid chromatography (HPLC) and gas chromatography-mass spectrometry (GC-MS) are used to detect and quantify Ketorolac EP Impurity J. These techniques are essential for monitoring the quality of ketorolac and ensuring it is safe for human consumption.
In conclusion, Ketorolac EP Impurity J is an impurity found in the synthesis of ketorolac. Its chemical information is important for quality control purposes, and analytical techniques are used to detect and quantify it.
Get an Instant Quote
Related Compounds
Ketorolac EP Impurity I | N-Nitroso Ketorolac 2-Benzoylpyrrole Impurity | Ketorolac 1-Keto Analog | N-(1,3-dihydroxy-2-(hydroxymethyl)propan-2-yl)-N-methylnitrous amide | Ketorolac EP Impurity H | Ketorolac tromethamine | Ketorolac EP Impurity F | Ketorolac EP Impurity B | N-(1,3-dihydroxy-2-(hydroxymethyl)propan-2-yl)-N-hydroxynitrous amide | Ketorolac EP Impurity A | N-Nitroso Ketorolac Triethylester Intermediate | Ketorolac EP Impurity C | Ketorolac EP Impurity G | Ketorolac EP Impurity E | Ketorolac EP Impurity D | Bromo ketorolac |