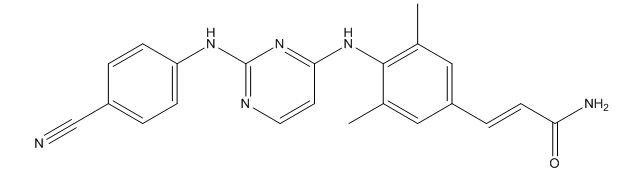
Rilpivirine amide 1 impurity
| Product Name | Rilpivirine amide 1 impurity |
|---|---|
| Alternate Names | Rilpivirine Impurities, Impurities of Rilpivirine |
| CAT No. | CS-O-15232 |
| CAS No. | 500288-66-4 |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | 384.43 g/mol |
| Mol. For. | C₂₂H₂₀N₆O |
| Hazardous | This is a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Rilpivirine |
| Purity | >98% |
| Therapeutic | Antiretroviral / Anti-HIV |
| Smileys | CC1=C(C(C)=CC(/C=C/C(N)=O)=C1)NC2=NC(NC3=CC=C(C#N)C=C3)=NC=C2 |
| Canonical Smiles | CC1=CC(=CC(=C1NC2=NC(=NC=C2)NC3=CC=C(C=C3)C#N)C)C=CC(=O)N |
| InchIKey | LEOGADCYBSSOOZ-VMPITWQZSA-N |
| Inchl | InChI=1S/C22H20N6O/c1-14-11-17(5-8-19(24)29)12-15(2)21(14)27-20-9-10-25-22(28-20)26-18-6-3-16(13-23)4-7-18/h3-12H,1-2H3,(H2,24,29)(H2,25,26,27,28)/b8-5+ |
| IUPAC | (E)-3-[4-[[2-(4-cyanoanilino)pyrimidin-4-yl]amino]-3,5-dimethylphenyl]prop-2-enamide |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Rilpivirine amide 1 impurity is a synthetic organic compound that is used as a reference standard for analytical purposes in the pharmaceutical industry. It is a potential impurity that may be present in Rilpivirine, an antiviral medication used for the treatment of HIV-1 infection.
Chemically, Rilpivirine amide 1 impurity is an amide derivative of Rilpivirine that is formed during the synthesis of the drug. It has the molecular formula C22H18F3N5O2 and a molecular weight of 447.4 g/mol. The compound is a white to off-white powder that is soluble in organic solvents like methanol and DMSO.
Rilpivirine amide 1 impurity is used as a reference standard in the development and validation of analytical methods to detect and quantify impurities in Rilpivirine. It is also used in quality control to ensure the purity and potency of the drug substance and finished product.
The presence of impurities in pharmaceuticals can affect their safety and efficacy. Thus, it is essential to monitor and control impurities in Rilpivirine to ensure that it is safe for use. Rilpivirine amide 1 impurity is an important tool in this process, providing accurate and reliable analytical data for the pharmaceutical industry.
Get an Instant Quote
Related Compounds
Rilpivirine Glycosamine product-I | Rilpivirine Impurity 13 | Rilpivirine Amadori Rearrangement product-II | Rilpivirine Methyl ester impurity | Rilpivirine Impurity 10 Di Hcl salt | Dinitroso Rilpivirine | Rilpivirine Impurity 2 | Rilpivirine Nitrile Desmethyl impurity | Rilpivirine Impurity 7 | Rilpivirine amide 2 impurity | Rilpivirine Keto Impurity | Rilpivirine Glycosamine product-II | Rilpivirine Amadori Rearrangement product-I |