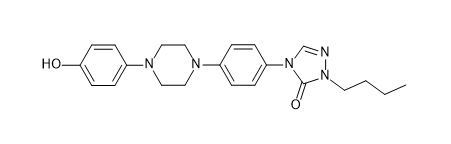
Itraconazole Hydroxy Butyltriazolone Impurity
| Product Name | Itraconazole Hydroxy Butyltriazolone Impurity |
|---|---|
| Alternate Names | Itraconazole Impurities, Impurities of Itraconazole |
| CAT No. | CS-O-15442 |
| CAS No. | 89848-20-4 |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | 393.48 g/mol |
| Mol. For. | C₂₂H₂₇N₅O₂ |
| Hazardous | This is not a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Itraconazole |
| Purity | >98% |
| Therapeutic | Anti-Fungals |
| Smileys | O=C1N(C2=CC=C(N3CCN(C4=CC=C(O)C=C4)CC3)C=C2)C=NN1CCCC |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Itraconazole Hydroxy Butyltriazolone Impurity is a chemical compound that is used in the pharmaceutical industry. It is an impurity that is commonly found in Itraconazole, which is a medication used to treat fungal infections. The presence of this impurity can affect the quality and effectiveness of the medication, which is why it is important to monitor and control its levels.
Chemically, Itraconazole Hydroxy Butyltriazolone Impurity is a triazole compound that has a hydroxy butyl group attached to it. It is formed as a byproduct during the synthesis of Itraconazole and can be present in varying amounts depending on the manufacturing process. The impurity is known to affect the stability of the medication and can lead to degradation over time, which can result in reduced potency and efficacy.
To ensure the quality and safety of the medication, regulatory agencies such as the US Food and Drug Administration (FDA) have set limits on the amount of Itraconazole Hydroxy Butyltriazolone Impurity that is allowed in Itraconazole products. Manufacturers must adhere to these regulations and conduct regular testing to ensure that their products meet these standards.
In conclusion, Itraconazole Hydroxy Butyltriazolone Impurity is an important compound to monitor in the pharmaceutical industry, particularly in the production of Itraconazole medications. By controlling its levels and adhering to regulatory standards, manufacturers can ensure the quality and efficacy of their products.
Get an Instant Quote
Related Compounds
Itraconazole Methoxy Propyltriazolone Impurity | Itraconazole Epimer | ITRACONAZOLE IMPURITY 18 | Itraconazole EP Impurity C | Itraconazole EP Impurity A | Itraconazole Desethylene-seco-piperazine Di-N-formyl Impurity | itraconazole 4-Triazolyl isomer | Itraconazole Impurity 7 | ITRACONAZOLE RELATED IMPURITY 3 | Itraconazole Methoxy Isopropyltriazolone Impurity | itraconazole n-Butyl isomer | N-Nitroso Itraconazole Impurity 1 | ITRACONAZOLE IMPURITY 13 | Itraconazole S,R IMPURITY | ITRACONAZOLE RELATED IMPURITY 1 | ITRACONAZOLE IMPURITY 11 | Itraconazole Methoxy Hydrazinyl Impurity | Itraconazole EP Impurity G | Itraconazole Hydroxy Propyltriazolone Impurity | Itraconazole EP Impurity F | Itraconazole EP Impurity D | Itraconazole Impurity 2 | Itraconazole EP Impurity B | Itraconazole Hydroxy Isopropyltriazolone Impurity | ITRACONAZOLE n-propyl impurity | Itraconazole Didioxolanyl analog | Itraconazole EP Impurity E | ITRACONAZOLE IMPURITY 19 |