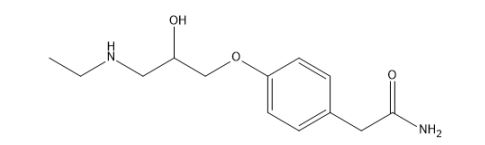
Atenolol EP Impurity I
| Chemical Name | Atenolol EP Impurity I |
|---|---|
| Alternate Names | Atenolol Impurities, Impurities of Atenolol |
| CAT No. | CS-O-16145 |
| CAS Registry# | 1797116-92-7 |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | 252.3 g/mol |
| Mol. For. | C13H20N2O3 |
| Hazardous | This is a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Atenolol |
| Therapeutic | Anti-Migraines |
| Smileys | CCNCC(COC1=CC=C(C=C1)CC(=O)N)O |
| Canonical Smiles | CCNCC(COC1=CC=C(C=C1)CC(=O)N)O |
| InchIKey | BXEVWKYWEMQUHC-UHFFFAOYSA-N |
| Inchl | InChI=1S/C13H20N2O3/c1-2-15-8-11(16)9-18-12-5-3-10(4-6-12)7-13(14)17/h3-6,11,15-16H,2,7-9H2,1H3,(H2,14,17) |
| IUPAC | 2-[4-[3-(ethylamino)-2-hydroxypropoxy]phenyl]acetamide |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Atenolol EP Impurity I is a chemical compound that is used as a reference standard for research and development purposes. It is a known impurity that is commonly used in the pharmaceutical industry to help determine the purity of atenolol, a beta-blocker medication that is used to treat high blood pressure, angina, and other cardiovascular conditions.
The chemical structure of Atenolol EP Impurity I is similar to that of atenolol, but contains an additional functional group that distinguishes it from the parent compound. This impurity is typically synthesized in a laboratory setting using a variety of chemical reactions and techniques, and is then purified through various chromatography methods.
It is important to note that Atenolol EP Impurity I is not intended for human consumption, as its effects on the body are largely unknown. Rather, it is used as a tool for analytical and research purposes, such as in the development of new drugs or in the testing of existing pharmaceuticals for impurities.
Overall, Atenolol EP Impurity I plays an important role in the pharmaceutical industry by providing scientists and researchers with a reliable reference standard for the analysis and characterization of atenolol and related compounds.
Get an Instant Quote
Related Compounds
N-Nitroso-Atenolol EP Impurity H | Atenolol EP Impurity G (sodium salt) | Atenolol Impurity Standard BP | Atenolol EP Impurity G | N-Nitroso Atenolol-D7 | Atenolol Impurity H | Atenolol EP Impurity G | Atenolol EP Impurity F | N-FORMYL ATENOLOL | Atenolol EP Impurity D | Atenolol EP Impurity B | Atenolol EP Impurity C | Atenolol Blocker Acid Impurity | N-Nitroso-Atenolol EP Impurity G | Atenolol EP impurity E | Hydroxyatenolol | R Atenolol |