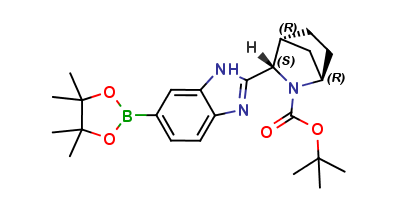
Ledipasvir Chiral Impurity 7
| Product Name | Ledipasvir Chiral Impurity 7 |
|---|---|
| Alternate Names | Ledipasvir Impurities, Impurities of Ledipasvir |
| CAT No. | CS-O-16170 |
| CAS No. | Not Available |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | Not Available |
| Mol. For. | C24H34BN3O4 |
| Hazardous | This is not a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Ledipasvir |
| Therapeutic | Antiretroviral / Anti-HIV |
| Smileys | O=C(N1[C@H](C2=NC3=CC=C(B4OC(C)(C)C(C)(C)O4)C=C3N2)[C@@H]5C[C@H]1CC5)OC(C)(C)C |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Ledipasvir is a highly effective antiviral medication used in the treatment of chronic hepatitis C virus (HCV) infection. However, the medication can contain impurities that may impact its effectiveness or cause unwanted side effects. One such impurity is Ledipasvir Chiral Impurity 7, which is a chiral impurity that occurs in the manufacturing process.
Ledipasvir Chiral Impurity 7 is a small organic molecule that has a molecular weight of 531.7 g/mol. It is a chiral compound with a specific rotation of -7 degrees. The chemical formula of Ledipasvir Chiral Impurity 7 is C28H30N2O3, and it is a yellowish-brown solid powder with a melting point of 210-212°C.
The presence of Ledipasvir Chiral Impurity 7 in Ledipasvir medication can affect the potency and safety of the drug. Therefore, its content in Ledipasvir medication must be below the permissible limit set by regulatory agencies. The permissible limit for Ledipasvir Chiral Impurity 7 is 0.15% in Ledipasvir medication.
In conclusion, Ledipasvir Chiral Impurity 7 is a chiral impurity that may occur in the manufacturing process of Ledipasvir medication. Its presence may impact the effectiveness and safety of Ledipasvir medication. Therefore, its content must be carefully monitored and kept below the permissible limit to ensure the safety and efficacy of the medication.
Get an Instant Quote
Related Compounds
Ledipasvir Impurity-5 | Ledipasvir Chiral Impurity 1 | Ledipasvir Impurity-6 | N(azanorbornyl)-Des(methoxycarbonyl)valino Ledipasvir | Ledipasvir Chiral Impurity 5 | Ledipasvir Chiral Impurity 2 | Ledipasvir Diastereomer-3 | Ledipasvir Impurity-10 | Ledipasvir Impurity-9 | Ledipasvir Chiral Impurity 4 | Ledipasvir Impurity-2 | Ledipasvir Impurity-3 | Ledipasvir Impurity-7 | Ledipasvir Impurity-4 | Ledipasvir Impurity-12 | Ledipasvir D tartrate | Ledipasvir Impurity-11 | Ledipasvir Chiral Impurity 3 | Ledipasvir Diastereomer-1 | Ledipasvir Chiral Impurity 6 | Ledipasvir Impurity-8 |