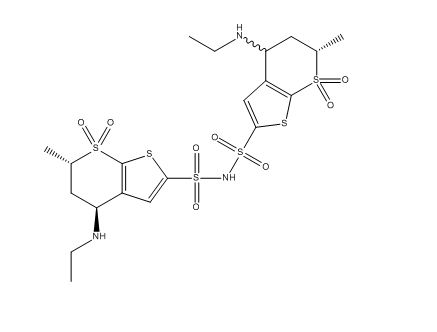
Dorzolamide Impurity 4 (mixture of diastereomers)
| Chemical Name | Dorzolamide Impurity 4 (mixture of diastereomers) |
|---|---|
| Alternate Names | Dorzolamide Impurities, Impurities of Dorzolamide |
| CAT No. | CS-O-16392 |
| CAS Registry# | Not Available |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | 631.85 g/mol |
| Mol. For. | C₂₀H₂₉N₃O₈S₆ |
| Hazardous | This is a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Dorzolamide |
| Purity | 95% |
| Smileys | O=[S]1(C2=C([C@H](C[C@@H]1C)NCC)C=C(S(=O)(NS(C3=CC(C(C[C@@H]4C)NCC)=C([S]4(=O)=O)S3)(=O)=O)=O)S2)=O |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Dorzolamide Impurity 4 is a mixture of diastereomers that is commonly used in the pharmaceutical industry as an impurity standard for the analysis and identification of Dorzolamide hydrochloride, a carbonic anhydrase inhibitor used in the treatment of glaucoma. This impurity is not intended for use in humans or animals, but rather as a reference standard for analytical purposes.
Chemically, Dorzolamide Impurity 4 is a mixture of two diastereomers, which are stereoisomers that are not mirror images of each other. These diastereomers have different physical and chemical properties, including different melting points, boiling points, and solubilities. The mixture can be identified using chromatographic techniques such as high-performance liquid chromatography (HPLC) or gas chromatography (GC).
In order to ensure the safety and efficacy of pharmaceutical products, regulatory bodies such as the United States Pharmacopeia (USP) and the European Pharmacopoeia (EP) require that impurities such as Dorzolamide Impurity 4 be identified and quantified in drug products. This helps to ensure that the drug product is of high quality and meets the required standards for safety and efficacy.
Overall, Dorzolamide Impurity 4 is an important reference standard used in the pharmaceutical industry for the analysis and identification of Dorzolamide hydrochloride, and is an essential tool in ensuring the safety and efficacy of pharmaceutical products.
Get an Instant Quote
Related Compounds
N-Acetyl Dorzolamide | Dorzolamide Impurity B | N-Nitroso-Dorzolamide | Dorzolamide Desaminosulfonyl HCl | rac-trans Dorzolamide | Dorzolamide Hydrochloride impurity 3 | Dorzolamide EP Impurity B HCl salt | Dorzolamide EP Impurity B | Dorzolamide Maleic Acid Adduct | Dorzolamide USP Related Compound A | Dorzolamide EP Impurity C | ent-Dorzolamide | Dorzolamide Impurity A Hydrochloride |