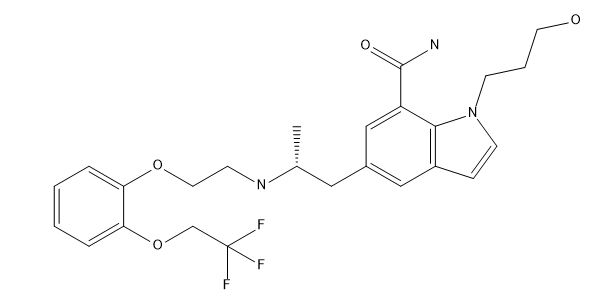
Silodosin Dehydro Impurity
| Product Name | Silodosin Dehydro Impurity |
|---|---|
| Alternate Names | Silodosin Impurities, Impurities of Silodosin |
| CAT No. | CS-O-31066 |
| CAS No. | 175870-21-0 |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | 493.52 g/mol |
| Mol. For. | C₂₅H₃₀F₃N₃O₄ |
| Hazardous | This is a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Silodosin |
| Purity | 95% |
| Therapeutic | Hormones |
| Smileys | CC(CC1=CC(=C2C(=C1)C=CN2CCCO)C(=O)N)NCCOC3=CC=CC=C3OCC(F)(F)F |
| Canonical Smiles | CC(CC1=CC(=C2C(=C1)C=CN2CCCO)C(=O)N)NCCOC3=CC=CC=C3OCC(F)(F)F |
| InchIKey | VICSLOHTZDWOFF-QGZVFWFLSA-N |
| Inchl | InChI=1S/C25H30F3N3O4/c1-17(30-8-12-34-21-5-2-3-6-22(21)35-16-25(26,27)28)13-18-14-19-7-10-31(9-4-11-32)23(19)20(15-18)24(29)33/h2-3,5-7,10,14-15,17,30,32H,4,8-9,11-13,16H2,1H3,(H2,29,33)/t17-/m1/s1 |
| IUPAC | 1-(3-hydroxypropyl)-5-[(2R)-2-[2-[2-(2,2,2-trifluoroethoxy)phenoxy]ethylamino]propyl]indole-7-carboxamide |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Silodosin Dehydro Impurity is a chemical compound that is used in the pharmaceutical industry as a reference standard for quality control testing of Silodosin drug products. It is a synthetic impurity that is produced as a by-product during the manufacturing process of Silodosin. This impurity is of great importance as it can affect the potency and efficacy of the drug product.
Silodosin itself is an alpha-adrenergic receptor antagonist that is used to treat the symptoms of benign prostatic hyperplasia (BPH) in men. It works by relaxing the muscles in the prostate gland and bladder neck, which improves urine flow and reduces the symptoms associated with BPH.
The chemical structure of Silodosin Dehydro Impurity has been elucidated and its properties have been characterized using advanced analytical techniques such as high-performance liquid chromatography (HPLC), gas chromatography-mass spectrometry (GC-MS), and nuclear magnetic resonance (NMR) spectroscopy.
It is important for pharmaceutical manufacturers to monitor the levels of Silodosin Dehydro Impurity in their drug products to ensure that they are within acceptable limits. This is because high levels of impurities can lead to adverse side effects or decreased efficacy of the drug product. Therefore, Silodosin Dehydro Impurity is a critical component in the quality control testing of Silodosin drug products.
Get an Instant Quote
Related Compounds
Silodosin Nitrile Impurity | Benzyl amine silodosin | Silodosin Related Comp B | N-Nitroso Benzoyl Silodosin impurity | Silodosin Impurity 7 DiHCl | Dehydro Silodosin KSM-1 | Silodosin Depropanol impurity | Benzyl Silodosin | Silodosin (S-ISOMER) | Silodosin Impurity 2 HCl | Silodosin Impurity 3 (Dimer Impurity) | N-Nitroso Silodosin impurity | silodosin KSM I Carbonitrile Impurity 1 | Deshydroxy propyl Dehydro Silodosin | Dimer Impurity of Silodosin Stage-I | O-Acetyl Silodosin | Silodosin Impurity B | Silodosin Impurity 14 | N-tert-Butyloxycarbonyl Dehydro Silodosin | Racemic Stage I of Silodosin |