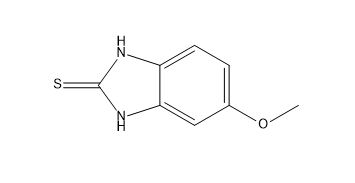
Omeprazole EP Impurity A
| Product Name | Omeprazole EP Impurity A |
|---|---|
| Alternate Names | Omeprazole Impurities, Impurities of Omeprazole |
| CAT No. | CS-O-31075 |
| CAS No. | 37052-78-1 |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | 180.23 g/mol |
| Mol. For. | C₈H₈N₂OS |
| Hazardous | This is a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Omeprazole |
| Purity | >98% |
| Therapeutic | Anti ulcer |
| Smileys | COC1=CC2=C(C=C1)NC(=S)N2 |
| Canonical Smiles | COC1=CC2=C(C=C1)NC(=S)N2 |
| InchIKey | KOFBRZWVWJCLGM-UHFFFAOYSA-N |
| Inchl | InChI=1S/C8H8N2OS/c1-11-5-2-3-6-7(4-5)10-8(12)9-6/h2-4H,1H3,(H2,9,10,12) |
| IUPAC | 5-methoxy-1,3-dihydrobenzimidazole-2-thione |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Omeprazole EP Impurity A is a chemical compound that is commonly used in the pharmaceutical industry as an impurity standard for the analysis of omeprazole. Omeprazole is a proton pump inhibitor drug that is used to reduce the amount of acid produced in the stomach. Omeprazole EP Impurity A is a degradation product of omeprazole that is formed during the manufacturing process or during storage of the drug.
The chemical formula of Omeprazole EP Impurity A is C17H19N3O3S, and its molecular weight is 345.42 g/mol. It is a white to off-white crystalline powder that is soluble in methanol, ethanol, and acetonitrile. Omeprazole EP Impurity A is a chiral compound, and it exists as a racemic mixture of two enantiomers.
The usage of Omeprazole EP Impurity A is crucial in the pharmaceutical industry as it helps in the identification and quantification of omeprazole in drug formulations. It is also used as a reference standard for the analysis of omeprazole in biological fluids such as plasma and urine.
In conclusion, Omeprazole EP Impurity A is a valuable analytical tool for the pharmaceutical industry. It is used to ensure the quality and purity of omeprazole-containing drugs and to monitor the stability of these drugs during storage.
Get an Instant Quote
Related Compounds
Omeprazole Isomer-2 5-Methoxy -1-[(4-methoxy-3,5-dimethyl-2-pyridinyl)]-0[2-(4-methoxy-3,5-dimethyl -2-pyridinyl)methyl]-sulfanyl]-1H-banzimidole | Omeprazole Impurity 63 | Omeprazole D13 | Omeprazole impurity D | Omeprazole Impurity 60 | Omeprazole EP Impurity C | Bis-Desmethoxy Omeprazole Sulfide | Omeprazole Hydrolysis Impurity | Omeprazole EP impurity F & G Mixture | N-Methyl Omeprazole (Mixture of isomers with the methylated nitrogens of imidazole) | Omeprazole Impurity 24 | N-Nitroso Omeprazole sodium | Omeprazole Impurity- K | Omeprazole Acid Methyl Ester Sulfide | Omeprazole Related Compound 5 | N-Nitroso Omeprazole EP Impurity I | Omeprazole sulfide 5-carboxylic acid | Omeprazole EP impurity I | o-Toluoyl-5-hydroxy Omeprazole Sulfide | Desulfoxide 4-Demethyl Omeprazole | N-Methyl Esomeprazole Isomer-1 | Omeprazole D15 | N-(4-Methoxy-3,5-dimethyl-2-pyridinyl)methyl Omeprazole | O,O-Didesmethyl Omeprazole | Omeprazole impurity (DIMER Mixture) | Omeprazole Impurity 25 | Omeprazole dibenzimidazole Impurity | Omeprazole Impurity 30 | Omeprazole EP Impurity F | Omeprazole O-hydrogen sulfite | N-methyl Omeprazole | Omeprazole-N-(S)-camphorsulfonamide | Omeprazole EP Impurity G | Omeprazole Related Compound 7 | Omeprazole Sulfide N1-Methyl 5-Methoxy Analog | Omeprazole Impurity 53 | Omeprazole Impurity 8 | N-Nitroso Omeprazole Sulfide | Omeprazole Impurity 79 | Omeprazole EP impurity B |