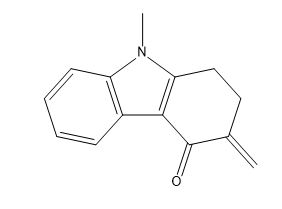
Ondansetron Impurity D
| Product Name | Ondansetron Impurity D |
|---|---|
| Alternate Names | Ondansetron Impurities, Impurities of Ondansetron |
| CAT No. | CS-O-31137 |
| CAS No. | 99614-64-9 |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | 211.26 g/mol |
| Mol. For. | C₁₄H₁₃NO |
| Hazardous | This is a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Ondansetron |
| Purity | 95% |
| Therapeutic | Anti-Cancer / Oncology |
| Smileys | O=C(C(CC1)=C)C2=C1N(C)C3=CC=CC=C23 |
| Canonical Smiles | CN1C2=C(C3=CC=CC=C31)C(=O)C(=C)CC2 |
| InchIKey | AGQJDIDJKSFVTC-UHFFFAOYSA-N |
| Inchl | InChI=1S/C14H13NO/c1-9-7-8-12-13(14(9)16)10-5-3-4-6-11(10)15(12)2/h3-6H,1,7-8H2,2H3 |
| IUPAC | 9-methyl-3-methylidene-1,2-dihydrocarbazol-4-one |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Ondansetron Impurity D, also known as Ondansetron Related Compound-D, is a chemical impurity that is commonly found in pharmaceutical products that contain Ondansetron. Ondansetron is a medication that is used to prevent nausea and vomiting caused by chemotherapy, radiation therapy, and surgery. The presence of impurities in pharmaceutical products is a common occurrence due to various manufacturing processes involved in the production of the medication.
Ondansetron Impurity D is a byproduct of the synthesis of Ondansetron and is usually present in small amounts. However, the presence of impurities in pharmaceutical products can have detrimental effects on the efficacy and safety of the medication. Therefore, it is essential to monitor and control the levels of impurities in pharmaceutical products.
Chemically, Ondansetron Impurity D is a derivative of Ondansetron and is classified as a benzimidazole compound. It has a molecular weight of 309.37 g/mol and a molecular formula of C18H19N3O2. The impurity is usually detected and measured using high-performance liquid chromatography (HPLC) techniques.
In conclusion, Ondansetron Impurity D is a chemical impurity that is commonly found in pharmaceutical products containing Ondansetron. While it is usually present in small amounts, controlling the levels of impurities is essential to ensure the safety and efficacy of the medication. Understanding the chemical properties and monitoring the levels of impurities is crucial in the production and quality control of pharmaceutical products.
Get an Instant Quote
Related Compounds
Ondansetron EP Impurity G HCl | Ondansetron EP Impurity A | Ondansetron USP Related Compound A | Nitroso keto indole | Ondansetron HCl Imp. C | Ondansetron EP Impurity A | Ondansetron Resolution Mixture | Ondansetron Nitroso Impurity 1 | Ondansetron EP Impurity G | Ondansetron Dimer | Ondansetron N-Desmethyl maleate |