Ondansetron USP Related Compound A
| Product Name | Ondansetron USP Related Compound A |
|---|---|
| Alternate Names | Ondansetron Impurities, Impurities of Ondansetron |
| CAT No. | CS-O-31138 |
| CAS No. | 119812-29-2 |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | 292.80 g/mol |
| Mol. For. | C₁₆H₂₁ClN₂O |
| Hazardous | This is a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Ondansetron |
| Purity | 95% |
| Therapeutic | Anti-Cancer / Oncology |
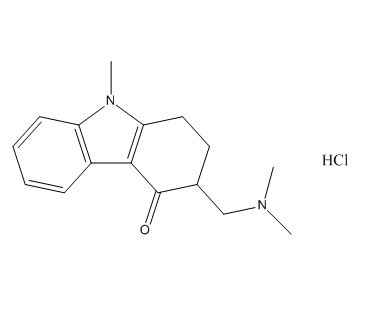
| Smileys | CN1C2=C(C3=CC=CC=C31)C(=O)C(CC2)CN(C)C.Cl |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Ondansetron USP Related Compound A is a reference standard used in the pharmaceutical industry to ensure the quality and purity of ondansetron, a medication commonly used to prevent nausea and vomiting caused by chemotherapy, radiation therapy, and surgery. Ondansetron USP Related Compound A is a synthetic impurity that is structurally related to ondansetron and is used as a marker in analytical testing to determine the amount of impurities present in ondansetron.
The chemical name of Ondansetron USP Related Compound A is (2RS,3RS)-3-[(1-methyl-1H-imidazol-5-yl)methyl]-2-(1H-tetrazol-5-yl)imidazo[1,2-b]pyridazine. Its molecular formula is C14H15N9 and its molecular weight is 333.34 g/mol. Ondansetron USP Related Compound A is a white to off-white crystalline powder that is soluble in water, ethanol, and dimethyl sulfoxide.
The usage of Ondansetron USP Related Compound A is governed by the United States Pharmacopeia (USP) and other regulatory bodies. It is used as a reference standard to ensure that ondansetron meets the required standards of purity and quality. Pharmaceutical manufacturers use Ondansetron USP Related Compound A in analytical testing during the development and production of ondansetron to ensure that it is safe and effective for use in patients.
Get an Instant Quote
Related Compounds
Ondansetron EP Impurity G HCl | Ondansetron EP Impurity A | Ondansetron N-Desmethyl maleate | Ondansetron Dimer | Ondansetron Resolution Mixture | Ondansetron HCl Imp. C | Ondansetron EP Impurity A | Ondansetron EP Impurity G | Nitroso keto indole | Ondansetron Impurity D | Ondansetron Nitroso Impurity 1 |