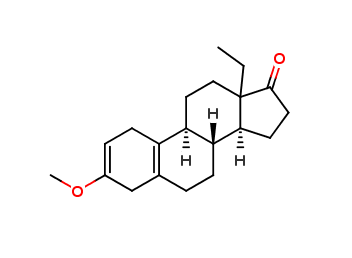
Levonorgestrel EP Impurity R
| Product Name | Levonorgestrel EP Impurity R |
|---|---|
| Alternate Names | Levonorgestrel Impurities, Impurities of Levonorgestrel |
| CAT No. | CS-O-32358 |
| CAS No. | 2322-77-2 |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | 300.44 g/mol |
| Mol. For. | C20H28O2 |
| Hazardous | This is not a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Levonorgestrel |
| Purity | 87.84 |
| Smileys | CC[C@@]1(C2=O)[C@](CC2)([H])[C@@](CCC3=C4CC=C(OC)C3)([H])[C@]4([H])CC1 |
| Canonical Smiles | CCC12CCC3C(C1CCC2=O)CCC4=C3CC=C(C4)OC |
| InchIKey | PQMRKLSVUBRLLQ-XSYGEPLQSA-N |
| Inchl | InChI=1S/C20H28O2/c1-3-20-11-10-16-15-7-5-14(22-2)12-13(15)4-6-17(16)18(20)8-9-19(20)21/h5,16-18H,3-4,6-12H2,1-2H3/t16-,17-,18+,20+/m1/s1 |
| IUPAC | (8R,9S,13S,14S)-13-ethyl-3-methoxy-4,6,7,8,9,11,12,14,15,16-decahydro-1H-cyclopenta[a]phenanthren-17-one |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Levonorgestrel is a synthetic progestin hormone used in various hormonal contraceptives. Levonorgestrel EP Impurity R is a known impurity that can be present in certain batches of levonorgestrel due to the synthesis process. This impurity has been identified as (17α)-13-Ethyl-17-hydroxy-18,19-dinorpregn-4-en-20-yn-3-one.
Although Levonorgestrel EP Impurity R is not an active ingredient in hormonal contraceptives, its presence can affect the quality and safety of the final product. Therefore, it is important to monitor its levels during the manufacturing process and ensure that it is within acceptable limits.
From a chemical perspective, Levonorgestrel EP Impurity R is a steroid compound with a molecular weight of 308.45 g/mol. Its structure contains a ketone functional group, a double bond, and a hydroxyl group, which are all common features in steroid molecules. The impurity can be detected and quantified using various analytical techniques such as high-performance liquid chromatography (HPLC) and gas chromatography-mass spectrometry (GC-MS).
In summary, Levonorgestrel EP Impurity R is a steroid impurity that can be present in levonorgestrel-based hormonal contraceptives. Its monitoring and control are essential to ensure the quality and safety of the final product.
Get an Instant Quote
Related Compounds
Levonorgestrel-3-isopropyldienol ether | Levonorgestrel EP Impurity Q | Levonorgestrel EP Impurity I | Levonorgestrel EP Impurity O | Levonorgestrel EP Impurity H | Levonorgestrel EP Impurity A | Levonorgestrel EP Impurity G | Levonorgestrel Glucuronide | Levonorgestrel Acetate Delta-5 Impurity | Levonorgestrel EP Impurity D | Levonorgestrel EP Impurity C | Levonorgestrel EP Impurity J | Levonorgestrel EP Impurity P | Levonorgestrel EP Impurity B |