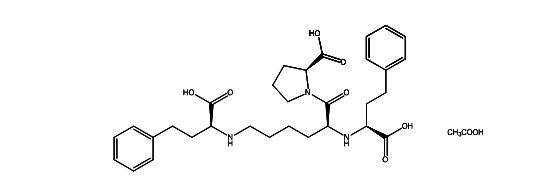
Lisinopril EP Impurity I Acetate salt
| Product Name | Lisinopril EP Impurity I Acetate salt |
|---|---|
| Alternate Names | Lisinopril Impurities, Impurities of Lisinopril |
| CAT No. | CS-O-33337 |
| CAS No. | 927819-64-5 |
| Category | Impurities |
| Stock | IN-Stock |
| Mol. Wt. | 567.7 g/mol |
| Mol. For. | C31H41N3O7 |
| Hazardous | This is a Hazardous Compound |
| COA | View Sample COA |
| MSDS | View Sample MSDS |
| Parent API | Lisinopril |
| Therapeutic | Anti-Hypertensives |
| Smileys | O=C([C@H]1N(C([C@@H](N[C@H](C(O)=O)CCC2=CC=CC=C2)CCCCN[C@H](C(O)=O)CCC3=CC=CC=C3)=O)CCC1)O |
| Canonical Smiles | C1CC(N(C1)C(=O)C(CCCCNC(CCC2=CC=CC=C2)C(=O)O)NC(CCC3=CC=CC=C3)C(=O)O)C(=O)O |
| InchIKey | MKOWVLMGJKGFMF-MBKURTIGSA-N |
| Inchl | InChI=1S/C31H41N3O7/c35-28(34-21-9-15-27(34)31(40)41)24(33-26(30(38)39)19-17-23-12-5-2-6-13-23)14-7-8-20-32-25(29(36)37)18-16-22-10-3-1-4-11-22/h1-6,10-13,24-27,32-33H,7-9,14-21H2,(H,36,37)(H,38,39)(H,40,41)/t24-,25?,26-,27-/m0/s1 |
| IUPAC | (2S)-1-[(2S)-2-[[(1S)-1-carboxy-3-phenylpropyl]amino]-6-[(1-carboxy-3-phenylpropyl)amino]hexanoyl]pyrrolidine-2-carboxylic acid |
| Controlled | No |
| Shipping | Free for purchase above 1000$ |
| Delivery | In-Stock, products will be dispatched within 24 hours via FedEx for USA, Europe, and other countries. |
| Return | Returns/replacement accepted if you are not satisfied with the quality of the product, (please send us an email with the reason/issues which are facing, within 15 days, after receipt of the product). |
| Ordering | Place your order online or by email sales@clearsynth.com |
Lisinopril EP Impurity I, also known as (2S)-1-[(2S,5S)-2,5-dimethyl-4-oxo-1,3-thiazolidin-3-yl]propan-2-one, is a chemical compound that is commonly used in the pharmaceutical industry as a reference standard for the analysis of Lisinopril, a medication used to treat high blood pressure and heart failure. Lisinopril EP Impurity I is a colorless to pale yellow solid that is soluble in organic solvents like methanol and ethanol.
Chemically, Lisinopril EP Impurity I is a thiazolidinone derivative, which is a cyclic amide containing a sulfur atom and a nitrogen atom in the ring. This compound is synthesized through a multi-step process that involves the reaction of 2,5-dimethyl-1,3-thiazolane-4-carboxylic acid with acetic anhydride, followed by the addition of sodium borohydride and acetic acid.
Lisinopril EP Impurity I is important in the pharmaceutical industry because it is used as a reference standard to ensure the quality and purity of Lisinopril. It is also used in the development and validation of analytical methods for the quantification of Lisinopril in drug formulations. The use of this impurity and its reference standard is crucial in the production of safe and effective medications for patients.
Get an Instant Quote
Related Compounds
N-trifluoroacetyl Lisinopril Intermediate | Lisinopril-D4 | Lisinopril EP Impurity G | Lisinopril-D8 | Lisinopril EP Impurity F | N-Benzyloxycarbonyl (S)-Lisinopril | Lisinopril EP Impurity D | Lisinopril Des-Proline dimer - II | Lisinopril EP impurity C Acetate salt | N2-(1-Ethoxycarbonyl-3-oxo-3-phenylpropyl)-N6-trifluoroacetyl-L-lysine | N-Benzyloxycarbonyl Lisinopril Cyclohexyl Analogue Ethyl Methyl Diester | N-Benzyloxycarbonyl (S)-Lisinopril Ethyl Methyl Diester | Lisinopril EP Impurity J | N-(1-Carboxy-3-phenylpropyl)-S-lisinopril (Mixture of diastereomers) | Lisinopril EP Impurity E | Lisinopril EP Impurity A | Lisinopril Intermediate | Lisinopril SRS-Diastereomer | Lisinopril EP Impurity A |